Non-enzymatic Activity of the α-Tubulin Acetyltransferase αTAT Limits Synaptic Bouton Growth in Neurons
- PMID: 31928876
- PMCID: PMC7047862
- DOI: 10.1016/j.cub.2019.12.022
Non-enzymatic Activity of the α-Tubulin Acetyltransferase αTAT Limits Synaptic Bouton Growth in Neurons
Abstract
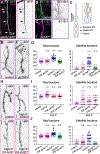
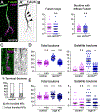
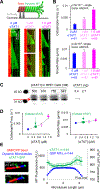
Neuronal axons terminate as synaptic boutons that form stable yet plastic connections with their targets. Synaptic bouton development relies on an underlying network of both long-lived and dynamic microtubules that provide structural stability for the boutons while also allowing for their growth and remodeling. However, a molecular-scale mechanism that explains how neurons appropriately balance these two microtubule populations remains a mystery. We hypothesized that α-tubulin acetyltransferase (αTAT), which both stabilizes long-lived microtubules against mechanical stress via acetylation and has been implicated in promoting microtubule dynamics, could play a role in this process. Using the Drosophila neuromuscular junction as a model, we found that non-enzymatic dαTAT activity limits the growth of synaptic boutons by affecting dynamic, but not stable, microtubules. Loss of dαTAT results in the formation of ectopic boutons. These ectopic boutons can be similarly suppressed by resupplying enzyme-inactive dαTAT or by treatment with a low concentration of the microtubule-targeting agent vinblastine, which acts to suppress microtubule dynamics. Biophysical reconstitution experiments revealed that non-enzymatic αTAT1 activity destabilizes dynamic microtubules but does not substantially impact the stability of long-lived microtubules. Further, during microtubule growth, non-enzymatic αTAT1 activity results in increasingly extended tip structures, consistent with an increased rate of acceleration of catastrophe frequency with microtubule age, perhaps via tip structure remodeling. Through these mechanisms, αTAT enriches for stable microtubules at the expense of dynamic ones. We propose that the specific suppression of dynamic microtubules by non-enzymatic αTAT activity regulates the remodeling of microtubule networks during synaptic bouton development.
Keywords: Drosophila; acetylation; microtubule; microtubule aging; neuromuscular junction; neuron; synaptic bouton; αTAT1.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures



Similar articles
-
dTip60 HAT activity controls synaptic bouton expansion at the Drosophila neuromuscular junction.PLoS One. 2011;6(10):e26202. doi: 10.1371/journal.pone.0026202. Epub 2011 Oct 27. PLoS One. 2011. PMID: 22046262 Free PMC article.
-
The hangover gene negatively regulates bouton addition at the Drosophila neuromuscular junction.Mech Dev. 2008 Aug;125(8):700-11. doi: 10.1016/j.mod.2008.04.004. Epub 2008 Apr 27. Mech Dev. 2008. PMID: 18524547
-
Tubulin acetyltransferase αTAT1 destabilizes microtubules independently of its acetylation activity.Mol Cell Biol. 2013 Mar;33(6):1114-23. doi: 10.1128/MCB.01044-12. Epub 2012 Dec 28. Mol Cell Biol. 2013. PMID: 23275437 Free PMC article.
-
The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation.Proc Natl Acad Sci U S A. 2010 Dec 14;107(50):21517-22. doi: 10.1073/pnas.1013728107. Epub 2010 Nov 10. Proc Natl Acad Sci U S A. 2010. PMID: 21068373 Free PMC article.
-
Embryonic and larval neural connectivity: progressive changes in synapse form and function at the neuromuscular junction mediated by cytoskeletal regulation.Wiley Interdiscip Rev Dev Biol. 2013 Nov-Dec;2(6):747-65. doi: 10.1002/wdev.114. Epub 2013 Mar 15. Wiley Interdiscip Rev Dev Biol. 2013. PMID: 24123935 Review.
Cited by
-
α-Tubulin acetylation at lysine 40 regulates dendritic arborization and larval locomotion by promoting microtubule stability in Drosophila.PLoS One. 2023 Feb 24;18(2):e0280573. doi: 10.1371/journal.pone.0280573. eCollection 2023. PLoS One. 2023. PMID: 36827311 Free PMC article.
-
Acetylated α-tubulin K394 regulates microtubule stability to shape the growth of axon terminals.Curr Biol. 2022 Feb 7;32(3):614-630.e5. doi: 10.1016/j.cub.2021.12.012. Epub 2022 Jan 25. Curr Biol. 2022. PMID: 35081332 Free PMC article.
-
The α-tubulin acetyltransferase ATAT1: structure, cellular functions, and its emerging role in human diseases.Cell Mol Life Sci. 2024 Apr 23;81(1):193. doi: 10.1007/s00018-024-05227-x. Cell Mol Life Sci. 2024. PMID: 38652325 Free PMC article. Review.
References
-
- Mitchison T, and Kirschner M. (1984). Dynamic instability of microtubule growth. Nature 312, 237–242. - PubMed
-
- Chapin SJ, and Bulinski JC (1992). Microtubule stabilization by assembly-promoting microtubule-associated proteins: a repeat performance. Cell Motil Cytoskeleton 23, 236–243. - PubMed
-
- Gardner MK, Zanic M, Gell C, Bormuth V, and Howard J. (2011). Depolymerizing Kinesins Kip3 and MCAK Shape Cellular Microtubule Architecture by Differential Control of Catastrophe. Cell 147, 1092–1103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

