A Bistable Mechanism Mediated by Integrins Controls Mechanotaxis of Leukocytes
- PMID: 31928762
- PMCID: PMC7002925
- DOI: 10.1016/j.bpj.2019.12.013
A Bistable Mechanism Mediated by Integrins Controls Mechanotaxis of Leukocytes
Abstract
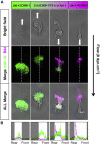
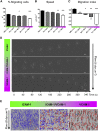
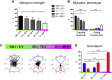
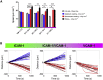
Recruitment of leukocytes from blood vessels to inflamed zones is guided by biochemical and mechanical stimuli, with the mechanisms only partially deciphered. Here, we studied the guidance by the flow of primary human effector T lymphocytes crawling on substrates coated with ligands of integrins lymphocyte function-associated antigen 1 (LFA-1) (αLβ2) and very late antigen 4 (VLA-4) (α4β1). We reveal that cells segregate in two populations of opposite orientation for combined adhesion and show that decisions of orientation rely on a bistable mechanism between LFA-1-mediated upstream and VLA-4-mediated downstream phenotypes. At the molecular level, bistability results from a differential front-rear polarization of both integrin affinities, combined with an inhibiting cross talk of LFA-1 toward VLA-4. At the cellular level, direction is determined by the passive, flow-mediated orientation of the nonadherent cell parts, the rear uropod for upstream migration, and the front lamellipod for downstream migration. This chain of logical events provides a comprehensive mechanism of guiding, from stimuli to cell orientation.
Copyright © 2019 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
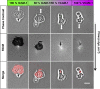

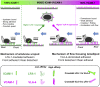
Similar articles
-
Integrin cross talk: activation of lymphocyte function-associated antigen-1 on human T cells alters alpha4beta1- and alpha5beta1-mediated function.J Cell Biol. 1997 Sep 22;138(6):1437-47. doi: 10.1083/jcb.138.6.1437. J Cell Biol. 1997. PMID: 9298996 Free PMC article.
-
Integrin crosstalk allows CD4+ T lymphocytes to continue migrating in the upstream direction after flow.Integr Biol (Camb). 2019 Dec 31;11(10):384-393. doi: 10.1093/intbio/zyz034. Integr Biol (Camb). 2019. PMID: 31851360 Free PMC article.
-
Modulation of integrin-mediated intercellular adhesion during the interaction of thymocytes with stromal cells expressing VLA-4 and LFA-1 ligands.Eur J Immunol. 1996 Sep;26(9):2050-5. doi: 10.1002/eji.1830260913. Eur J Immunol. 1996. PMID: 8814245
-
Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases.Med Res Rev. 2002 Mar;22(2):146-67. doi: 10.1002/med.10001. Med Res Rev. 2002. PMID: 11857637 Review.
-
Roles of adhesion molecules in T-cell recognition: fundamental similarities between four integrins on resting human T cells (LFA-1, VLA-4, VLA-5, VLA-6) in expression, binding, and costimulation.Immunol Rev. 1990 Apr;114:109-43. doi: 10.1111/j.1600-065x.1990.tb00563.x. Immunol Rev. 1990. PMID: 2196219 Review.
Cited by
-
Keratocytes migrate against flow with a roly-poly-like mechanism.Proc Natl Acad Sci U S A. 2022 Nov 29;119(48):e2210379119. doi: 10.1073/pnas.2210379119. Epub 2022 Nov 21. Proc Natl Acad Sci U S A. 2022. PMID: 36409912 Free PMC article.
-
Natural Killer Cell Mechanosensing in Solid Tumors.Bioengineering (Basel). 2024 Mar 28;11(4):328. doi: 10.3390/bioengineering11040328. Bioengineering (Basel). 2024. PMID: 38671750 Free PMC article. Review.
-
How integrin phosphorylations regulate cell adhesion and signaling.Trends Biochem Sci. 2022 Mar;47(3):265-278. doi: 10.1016/j.tibs.2021.11.003. Epub 2021 Dec 4. Trends Biochem Sci. 2022. PMID: 34872819 Free PMC article. Review.
-
LFA-1 Activation in T-Cell Migration and Immunological Synapse Formation.Cells. 2023 Apr 12;12(8):1136. doi: 10.3390/cells12081136. Cells. 2023. PMID: 37190045 Free PMC article. Review.
-
Leukocyte transmigration and longitudinal forward-thrusting force in a microfluidic Transwell device.Biophys J. 2021 Jun 1;120(11):2205-2221. doi: 10.1016/j.bpj.2021.03.037. Epub 2021 Apr 8. Biophys J. 2021. PMID: 33838136 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

