Clostridioides difficile Colonization Is Differentially Associated With Gut Microbiome Profiles by Infant Feeding Modality at 3-4 Months of Age
- PMID: 31921134
- PMCID: PMC6917614
- DOI: 10.3389/fimmu.2019.02866
Clostridioides difficile Colonization Is Differentially Associated With Gut Microbiome Profiles by Infant Feeding Modality at 3-4 Months of Age
Abstract
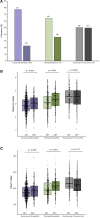
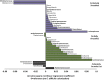
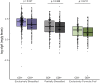
Colonization with Clostridioides difficile occurs in up to half of infants under the age of 3 months, is strongly influenced by feeding modality and is largely asymptomatic. In spite of this, C. difficile's presence has been associated with susceptibility to chronic disease later in childhood, perhaps by promoting or benefiting from changes in infant gut microbiome development, including colonization with pathogenic bacteria and disrupted production of microbial bioactive metabolites and proteins. In this study, the microbiomes of 1554 infants from the CHILD Cohort Study were described according to C. difficile colonization status and feeding mode at 3-4 months of age. C. difficile colonization was associated with a different gut microbiome profile in exclusively breastfed (EBF) vs. exclusively formula fed (EFF) infants. EBF infants colonized with C. difficile had an increased relative abundance of Firmicutes and Proteobacteria, decreased relative abundance of Bifidobacteriaceae, greater microbiota alpha-diversity, greater detectable fecal short chain fatty acids (SCFA), and lower detectable fecal secretory Immunoglobulin A (sIgA) than those not colonized. Similar but less pronounced differences were seen among partially breastfed infants (PBF) but EFF infants did not possess these differences in the gut microbiome according to colonization status. Thus, breastfed infants colonized with C. difficile appear to possess a gut microbiome that differs from non-colonized infants and resembles that of EFF infants, but the driving force and direction of this association remains unknown. Understanding these compositional differences as drivers of C. difficile colonization may be important to ensure future childhood health.
Keywords: Clostridioides difficile; SCFA; gut microbiota; infant feeding; metabolites; microbiome; sIgA.
Copyright © 2019 Drall, Tun, Morales-Lizcano, Konya, Guttman, Field, Mandal, Wishart, Becker, Azad, Lefebvre, Mandhane, Moraes, Sears, Turvey, Subbarao, Scott and Kozyrskyj.
Figures
Similar articles
-
Vitamin D supplementation in pregnancy and early infancy in relation to gut microbiota composition and C. difficile colonization: implications for viral respiratory infections.Gut Microbes. 2020 Nov 9;12(1):1799734. doi: 10.1080/19490976.2020.1799734. Gut Microbes. 2020. PMID: 32779963 Free PMC article.
-
Association of Exposure to Formula in the Hospital and Subsequent Infant Feeding Practices With Gut Microbiota and Risk of Overweight in the First Year of Life.JAMA Pediatr. 2018 Jul 2;172(7):e181161. doi: 10.1001/jamapediatrics.2018.1161. Epub 2018 Jul 2. JAMA Pediatr. 2018. PMID: 29868719 Free PMC article.
-
Rapid change of fecal microbiome and disappearance of Clostridium difficile in a colonized infant after transition from breast milk to cow milk.Microbiome. 2016 Oct 7;4(1):53. doi: 10.1186/s40168-016-0198-6. Microbiome. 2016. PMID: 27717398 Free PMC article.
-
Structural and functional changes within the gut microbiota and susceptibility to Clostridium difficile infection.Anaerobe. 2016 Oct;41:37-43. doi: 10.1016/j.anaerobe.2016.05.006. Epub 2016 May 12. Anaerobe. 2016. PMID: 27180006 Free PMC article. Review.
-
The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants' life: a systematic review.BMC Gastroenterol. 2016 Jul 30;16(1):86. doi: 10.1186/s12876-016-0498-0. BMC Gastroenterol. 2016. PMID: 27475754 Free PMC article. Review.
Cited by
-
Vitamin D supplementation in pregnancy and early infancy in relation to gut microbiota composition and C. difficile colonization: implications for viral respiratory infections.Gut Microbes. 2020 Nov 9;12(1):1799734. doi: 10.1080/19490976.2020.1799734. Gut Microbes. 2020. PMID: 32779963 Free PMC article.
-
Hen raising helps chicks establish gut microbiota in their early life and improve microbiota stability after H9N2 challenge.Microbiome. 2022 Jan 24;10(1):14. doi: 10.1186/s40168-021-01200-z. Microbiome. 2022. PMID: 35074015 Free PMC article.
-
Prenatal Depression, Breastfeeding, and Infant Gut Microbiota.Front Microbiol. 2021 Jul 30;12:664257. doi: 10.3389/fmicb.2021.664257. eCollection 2021. Front Microbiol. 2021. PMID: 34394021 Free PMC article.
-
Maternal smoking during pregnancy increases the risk of gut microbiome-associated childhood overweight and obesity.Gut Microbes. 2024 Jan-Dec;16(1):2323234. doi: 10.1080/19490976.2024.2323234. Epub 2024 Mar 4. Gut Microbes. 2024. PMID: 38436093 Free PMC article.
-
Delayed gut microbiota maturation in the first year of life is a hallmark of pediatric allergic disease.Nat Commun. 2023 Aug 29;14(1):4785. doi: 10.1038/s41467-023-40336-4. Nat Commun. 2023. PMID: 37644001 Free PMC article.

