Structure-activity relationship studies of four novel 4-aminopyridine K+ channel blockers
- PMID: 31919372
- PMCID: PMC6952366
- DOI: 10.1038/s41598-019-56245-w
Structure-activity relationship studies of four novel 4-aminopyridine K+ channel blockers
Abstract
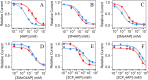
4-Aminopyridine (4AP) is a specific blocker of voltage-gated potassium channels (KV1 family) clinically approved for the symptomatic treatment of patients with multiple sclerosis (MS). It has recently been shown that [18F]3F4AP, a radiofluorinated analog of 4AP, also binds to KV1 channels and can be used as a PET tracer for the detection of demyelinated lesions in rodent models of MS. Here, we investigate four novel 4AP derivatives containing methyl (-CH3), methoxy (-OCH3) as well as trifluoromethyl (-CF3) in the 2 and 3 position as potential candidates for PET imaging and/or therapy. We characterized the physicochemical properties of these compounds (basicity and lipophilicity) and analyzed their ability to block Shaker K+ channel under different voltage and pH conditions. Our results demonstrate that three of the four derivatives are able to block voltage-gated potassium channels. Specifically, 3-methyl-4-aminopyridine (3Me4AP) was found to be approximately 7-fold more potent than 4AP and 3F4AP; 3-methoxy- and 3-trifluoromethyl-4-aminopyridine (3MeO4AP and 3CF34AP) were found to be about 3- to 4-fold less potent than 4AP; and 2-trifluoromethyl-4-AP (2CF34AP) was found to be about 60-fold less active. These results suggest that these novel derivatives are potential candidates for therapy and imaging.
Conflict of interest statement
The University of Chicago has obtained patents related to the compounds described here where P.B. is listed as inventor (U.S. Patent: US10160695B2 and US9617215B2). Other authors declare no competing interests.
Figures
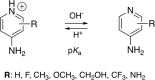


Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
"It Is a Big Spider Web of Things": Sensory Experiences of Autistic Adults in Public Spaces.Autism Adulthood. 2023 Dec 1;5(4):411-422. doi: 10.1089/aut.2022.0024. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116051 Free PMC article.
-
Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article.
-
Repetitive Negative Thinking As a Transdiagnostic Prospective Predictor of Depression and Anxiety Symptoms in Neurodiverse First-Semester College Students.Autism Adulthood. 2023 Dec 1;5(4):374-388. doi: 10.1089/aut.2022.0078. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116057 Free PMC article.
-
Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
Cited by
-
Neuroprotective Properties of 4-Aminopyridine.Neurol Neuroimmunol Neuroinflamm. 2021 Mar 2;8(3):e976. doi: 10.1212/NXI.0000000000000976. Print 2021 May. Neurol Neuroimmunol Neuroinflamm. 2021. PMID: 33653963 Free PMC article. Review.
-
Electro-steric opening of the CLC-2 chloride channel gate.Sci Rep. 2021 Jun 23;11(1):13127. doi: 10.1038/s41598-021-92247-3. Sci Rep. 2021. PMID: 34162897 Free PMC article.
-
Metabolic Stability of the Demyelination Positron Emission Tomography Tracer [18F]3-Fluoro-4-Aminopyridine and Identification of Its Metabolites.J Pharmacol Exp Ther. 2023 Jul;386(1):93-101. doi: 10.1124/jpet.122.001462. Epub 2023 Apr 6. J Pharmacol Exp Ther. 2023. PMID: 37024145 Free PMC article.
-
Radiochemical Synthesis and Evaluation of 3-[11C]Methyl-4-aminopyridine in Rodents and Nonhuman Primates for Imaging Potassium Channels in the CNS.ACS Chem Neurosci. 2022 Dec 7;13(23):3342-3351. doi: 10.1021/acschemneuro.2c00364. Epub 2022 Nov 23. ACS Chem Neurosci. 2022. PMID: 36417797 Free PMC article.
-
Syntheses of [11C]2- and [11C]3-trifluoromethyl-4-aminopyridine: potential PET radioligands for demyelinating diseases.RSC Med Chem. 2020 Jul 24;11(10):1161-1167. doi: 10.1039/d0md00190b. eCollection 2020 Oct 1. RSC Med Chem. 2020. PMID: 33479620 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

