Human pluripotent stem cell-derived chondroprogenitors for cartilage tissue engineering
- PMID: 31915836
- PMCID: PMC11104892
- DOI: 10.1007/s00018-019-03445-2
Human pluripotent stem cell-derived chondroprogenitors for cartilage tissue engineering
Abstract
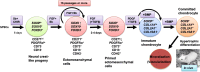
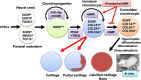
The cartilage of joints, such as meniscus and articular cartilage, is normally long lasting (i.e., permanent). However, once damaged, especially in large animals and humans, joint cartilage is not spontaneously repaired. Compensating the lack of repair activity by supplying cartilage-(re)forming cells, such as chondrocytes or mesenchymal stromal cells, or by transplanting a piece of normal cartilage, has been the basis of therapy for biological restoration of damaged joint cartilage. Unfortunately, current biological therapies face problems on a number of fronts. The joint cartilage is generated de novo from a specialized cell type, termed a 'joint progenitor' or 'interzone cell' during embryogenesis. Therefore, embryonic chondroprogenitors that mimic the property of joint progenitors might be the best type of cell for regenerating joint cartilage in the adult. Pluripotent stem cells (PSCs) are expected to differentiate in culture into any somatic cell type through processes that mimic embryogenesis, making human (h)PSCs a promising source of embryonic chondroprogenitors. The major research goals toward the clinical application of PSCs in joint cartilage regeneration are to (1) efficiently generate lineage-specific chondroprogenitors from hPSCs, (2) expand the chondroprogenitors to the number needed for therapy without loss of their chondrogenic activity, and (3) direct the in vivo or in vitro differentiation of the chondroprogenitors to articular or meniscal (i.e., permanent) chondrocytes rather than growth plate (i.e., transient) chondrocytes. This review is aimed at providing the current state of research toward meeting these goals. We also include our recent achievement of successful generation of "permanent-like" cartilage from long-term expandable, hPSC-derived ectomesenchymal chondroprogenitors.
Keywords: Differentiation; Endochondral ossification; Expansion; Growth factor; Mesenchymal; Permanent cartilage; Regeneration.
Figures


Similar articles
-
GDF5+ chondroprogenitors derived from human pluripotent stem cells preferentially form permanent chondrocytes.Development. 2022 Jun 1;149(11):dev196220. doi: 10.1242/dev.196220. Epub 2022 Jun 6. Development. 2022. PMID: 35451016 Free PMC article.
-
Comparative analysis of human bone marrow mesenchymal stem cells, articular cartilage derived chondroprogenitors and chondrocytes to determine cell superiority for cartilage regeneration.Acta Histochem. 2021 May;123(4):151713. doi: 10.1016/j.acthis.2021.151713. Epub 2021 Apr 21. Acta Histochem. 2021. PMID: 33894479
-
Reserve or Resident Progenitors in Cartilage? Comparative Analysis of Chondrocytes versus Chondroprogenitors and Their Role in Cartilage Repair.Cartilage. 2018 Apr;9(2):171-182. doi: 10.1177/1947603517736108. Epub 2017 Oct 19. Cartilage. 2018. PMID: 29047310 Free PMC article.
-
Cell sources for the regeneration of articular cartilage: the past, the horizon and the future.Int J Exp Pathol. 2012 Dec;93(6):389-400. doi: 10.1111/j.1365-2613.2012.00837.x. Epub 2012 Oct 18. Int J Exp Pathol. 2012. PMID: 23075006 Free PMC article. Review.
-
Rejuvenated Stem/Progenitor Cells for Cartilage Repair Using the Pluripotent Stem Cell Technology.Bioengineering (Basel). 2021 Apr 10;8(4):46. doi: 10.3390/bioengineering8040046. Bioengineering (Basel). 2021. PMID: 33920285 Free PMC article. Review.
Cited by
-
Directed differentiation of hPSCs through a simplified lateral plate mesoderm protocol for generation of articular cartilage progenitors.PLoS One. 2023 Jan 27;18(1):e0280024. doi: 10.1371/journal.pone.0280024. eCollection 2023. PLoS One. 2023. PMID: 36706111 Free PMC article.
-
Zone-Dependent Architecture and Biochemical Composition of Decellularized Porcine Nasal Cartilage Modulate the Activity of Adipose Tissue-Derived Stem Cells in Cartilage Regeneration.Int J Mol Sci. 2021 Sep 14;22(18):9917. doi: 10.3390/ijms22189917. Int J Mol Sci. 2021. PMID: 34576079 Free PMC article.
-
The Role of Chronic Inflammatory Bone and Joint Disorders in the Pathogenesis and Progression of Alzheimer's Disease.Front Aging Neurosci. 2020 Dec 7;12:583884. doi: 10.3389/fnagi.2020.583884. eCollection 2020. Front Aging Neurosci. 2020. PMID: 33364931 Free PMC article. Review.
-
Cartilage tissue engineering for craniofacial reconstruction.Arch Plast Surg. 2020 Sep;47(5):392-403. doi: 10.5999/aps.2020.01095. Epub 2020 Sep 15. Arch Plast Surg. 2020. PMID: 32971590 Free PMC article.
-
Craniofacial chondrogenesis in organoids from human stem cell-derived neural crest cells.iScience. 2024 Mar 28;27(4):109585. doi: 10.1016/j.isci.2024.109585. eCollection 2024 Apr 19. iScience. 2024. PMID: 38623327 Free PMC article.
References
-
- De Bari C, Roelofs AJ. Stem cell-based therapeutic strategies for cartilage defects and osteoarthritis. Curr Opin Pharmacol. 2018;40:74–80. - PubMed
-
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

