Coenzyme Q10 Regulation of Apoptosis and Oxidative Stress in H2O2 Induced BMSC Death by Modulating the Nrf-2/NQO-1 Signaling Pathway and Its Application in a Model of Spinal Cord Injury
- PMID: 31915512
- PMCID: PMC6930770
- DOI: 10.1155/2019/6493081
Coenzyme Q10 Regulation of Apoptosis and Oxidative Stress in H2O2 Induced BMSC Death by Modulating the Nrf-2/NQO-1 Signaling Pathway and Its Application in a Model of Spinal Cord Injury
Abstract
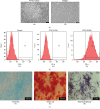
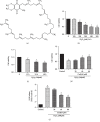
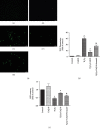
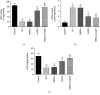
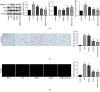
Spinal cord injury (SCI) has always been considered to be a devastating problem that results in catastrophic dysfunction, high disability rate, low mortality rate, and huge cost for the patient. Stem cell-based therapy, especially using bone marrow mesenchymal stem cells (BMSCs), is a promising strategy for the treatment of SCI. However, SCI results in low rates of cell survival and a poor microenvironment, which limits the therapeutic efficiency of BMSC transplantation. Coenzyme Q10 (CoQ10) is known as a powerful antioxidant, which inhibits lipid peroxidation and scavenges free radicals, and its combined effect with BMSC transplantation has been shown to have a powerful impact on protecting the vitality of cells, as well as antioxidant and antiapoptotic compounds in SCI. Therefore, we aimed to evaluate whether CoQ10 could decrease oxidative stress against the apoptosis of BMSCs in vitro and explored its molecular mechanisms. Furthermore, we investigated the protective effect of CoQ10 combined with BMSCs transplanted into a SCI model to verify its ability. Our results demonstrate that CoQ10 treatment significantly decreases the expression of the proapoptotic proteins Bax and Caspase-3, as shown through TUNEL-positive staining and the products of oxidative stress (ROS), while increasing the expression of the antiapoptotic protein Bcl-2 and the products of antioxidation, such as glutathione (GSH), against apoptosis and oxidative stress, in a H2O2-induced model. We also identified consistent results from the CoQ10 treatment of BMSCs transplanted into SCI rats in vivo. Moreover, the Nrf-2 signaling pathway was also investigated in order to detail its molecular mechanism, and the results show that it plays an important role, both in vitro and in vivo. Thus, CoQ10 exerts an antiapoptotic and antioxidant effect, as well as improves the microenvironment in vitro and in vivo. It may also protect BMSCs from oxidative stress and enhance their therapeutic efficiency when transplanted for SCI treatment.
Copyright © 2019 Xing Li et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures


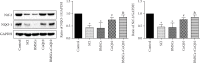
Similar articles
-
Tauroursodeoxycholic Acid Inhibited Apoptosis and Oxidative Stress in H2O2-Induced BMSC Death via Modulating the Nrf-2 Signaling Pathway: the Therapeutic Implications in a Rat Model of Spinal Cord Injury.Mol Neurobiol. 2024 Jul;61(7):3753-3768. doi: 10.1007/s12035-023-03754-5. Epub 2023 Nov 28. Mol Neurobiol. 2024. PMID: 38015303 Free PMC article.
-
Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival by inducing HIF-1α in injured neuronal cells derived exosomes culture system.Cell Death Dis. 2019 Feb 12;10(2):134. doi: 10.1038/s41419-019-1410-y. Cell Death Dis. 2019. PMID: 30755595 Free PMC article.
-
Coenzyme Q10 suppresses oxidative stress and apoptosis via activating the Nrf-2/NQO-1 and NF-κB signaling pathway after spinal cord injury in rats.Am J Transl Res. 2019 Oct 15;11(10):6544-6552. eCollection 2019. Am J Transl Res. 2019. PMID: 31737205 Free PMC article.
-
ROS: Executioner of regulating cell death in spinal cord injury.Front Immunol. 2024 Jan 23;15:1330678. doi: 10.3389/fimmu.2024.1330678. eCollection 2024. Front Immunol. 2024. PMID: 38322262 Free PMC article. Review.
-
Opposite Roles of Co-enzyme Q10 and Formaldehyde in Neurodegenerative Diseases.Am J Alzheimers Dis Other Demen. 2022 Jan-Dec;37:15333175221143274. doi: 10.1177/15333175221143274. Am J Alzheimers Dis Other Demen. 2022. PMID: 36455136 Free PMC article. Review.
Cited by
-
Modified Qing'e Formula protects against UV-induced skin oxidative damage via the activation of Nrf2/ARE defensive pathway.Front Pharmacol. 2022 Oct 28;13:976473. doi: 10.3389/fphar.2022.976473. eCollection 2022. Front Pharmacol. 2022. PMID: 36386207 Free PMC article.
-
Stability of Reduced and Oxidized Coenzyme Q10 in Finished Products.Antioxidants (Basel). 2021 Feb 27;10(3):360. doi: 10.3390/antiox10030360. Antioxidants (Basel). 2021. PMID: 33673604 Free PMC article.
-
Differential expression profiles of long noncoding RNAs and mRNAs in human bone marrow mesenchymal stem cells after exposure to a high dosage of dexamethasone.Stem Cell Res Ther. 2021 Jan 6;12(1):9. doi: 10.1186/s13287-020-02040-8. Stem Cell Res Ther. 2021. PMID: 33407832 Free PMC article.
-
Role and mechanism of ferroptosis in neurological diseases.Mol Metab. 2022 Jul;61:101502. doi: 10.1016/j.molmet.2022.101502. Epub 2022 Apr 18. Mol Metab. 2022. PMID: 35447365 Free PMC article. Review.
-
Effects of Coenzyme Q10 Supplementation on Biomarkers of Oxidative Stress in Adults: A GRADE-Assessed Systematic Review and Updated Meta-Analysis of Randomized Controlled Trials.Antioxidants (Basel). 2022 Jul 13;11(7):1360. doi: 10.3390/antiox11071360. Antioxidants (Basel). 2022. PMID: 35883851 Free PMC article. Review.
References
-
- Rivers C. S., Fallah N., Noonan V. K., et al. Health conditions: effect on function, health-related quality of life, and life satisfaction after traumatic spinal cord injury. A prospective observational registry cohort study. Archives of Physical Medicine and Rehabilitation. 2018;99(3):443–451. doi: 10.1016/j.apmr.2017.06.012. - DOI - PubMed
-
- Hiremath S. V., Hogaboom N. S., Roscher M. R., Worobey L. A., Oyster M. L., Boninger M. L. Longitudinal prediction of quality-of-life scores and locomotion in individuals with traumatic spinal cord injury. Archives of Physical Medicine and Rehabilitation. 2017;98(12):2385–2392. doi: 10.1016/j.apmr.2017.05.020. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

