The septate junction protein Mesh is required for epithelial morphogenesis, ion transport, and paracellular permeability in the Drosophila Malpighian tubule
- PMID: 31913700
- PMCID: PMC7099519
- DOI: 10.1152/ajpcell.00492.2019
The septate junction protein Mesh is required for epithelial morphogenesis, ion transport, and paracellular permeability in the Drosophila Malpighian tubule
Abstract
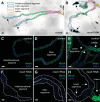
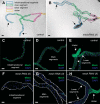
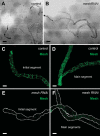
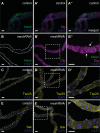
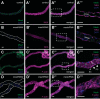
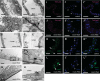
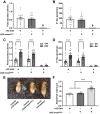
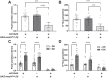
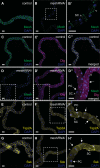
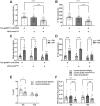
Septate junctions (SJs) are occluding cell-cell junctions that have roles in paracellular permeability and barrier function in the epithelia of invertebrates. Arthropods have two types of SJs, pleated SJs and smooth SJs (sSJs). In Drosophila melanogaster, sSJs are found in the midgut and Malpighian tubules, but the functions of sSJs and their protein components in the tubule epithelium are unknown. Here we examined the role of the previously identified integral sSJ component, Mesh, in the Malpighian tubule. We genetically manipulated mesh specifically in the principal cells of the tubule at different life stages. Tubules of flies with developmental mesh knockdown revealed defects in epithelial architecture, sSJ molecular and structural organization, and lack of urine production in basal and kinin-stimulated conditions, resulting in edema and early adult lethality. Knockdown of mesh during adulthood did not disrupt tubule epithelial and sSJ integrity but decreased the transepithelial potential, diminished transepithelial fluid and ion transport, and decreased paracellular permeability to 4-kDa dextran. Drosophila kinin decreased transepithelial potential and increased chloride permeability, and it stimulated fluid secretion in both control and adult mesh knockdown tubules but had no effect on 4-kDa dextran flux. Together, these data indicate roles for Mesh in the developmental maturation of the Drosophila Malpighian tubule and in ion and macromolecular transport in the adult tubule.
Keywords: Drosophila; Malpighian tubule; Mesh; drosokinin; paracellular permeability; smooth septate junctions.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
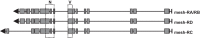


Similar articles
-
Molecular basis for epithelial morphogenesis and ion transport in the Malpighian tubule.Curr Opin Insect Sci. 2021 Oct;47:7-11. doi: 10.1016/j.cois.2021.02.001. Epub 2021 Feb 10. Curr Opin Insect Sci. 2021. PMID: 33581351 Free PMC article. Review.
-
The septate junction protein Tetraspanin 2A is critical to the structure and function of Malpighian tubules in Drosophila melanogaster.Am J Physiol Cell Physiol. 2020 Jun 1;318(6):C1107-C1122. doi: 10.1152/ajpcell.00061.2020. Epub 2020 Apr 8. Am J Physiol Cell Physiol. 2020. PMID: 32267718 Free PMC article.
-
A tetraspanin regulates septate junction formation in Drosophila midgut.J Cell Sci. 2016 Mar 15;129(6):1155-64. doi: 10.1242/jcs.180448. Epub 2016 Feb 4. J Cell Sci. 2016. PMID: 26848177
-
A novel protein complex, Mesh-Ssk, is required for septate junction formation in the Drosophila midgut.J Cell Sci. 2012 Oct 15;125(Pt 20):4923-33. doi: 10.1242/jcs.112243. Epub 2012 Aug 1. J Cell Sci. 2012. PMID: 22854041
-
Molecular dissection of smooth septate junctions: understanding their roles in arthropod physiology.Ann N Y Acad Sci. 2017 Jun;1397(1):17-24. doi: 10.1111/nyas.13366. Ann N Y Acad Sci. 2017. PMID: 28636800 Review.
Cited by
-
Combined transcriptome and proteome profiling reveal cell-type-specific functions of Drosophila garland and pericardial nephrocytes.Commun Biol. 2024 Nov 1;7(1):1424. doi: 10.1038/s42003-024-07062-z. Commun Biol. 2024. PMID: 39487357 Free PMC article.
-
Honey Bee Larval Hemolymph as a Source of Key Nutrients and Proteins Offers a Promising Medium for Varroa destructor Artificial Rearing.Int J Mol Sci. 2023 Aug 4;24(15):12443. doi: 10.3390/ijms241512443. Int J Mol Sci. 2023. PMID: 37569818 Free PMC article.
-
Drosophila melanogaster: a simple genetic model of kidney structure, function and disease.Nat Rev Nephrol. 2022 Jul;18(7):417-434. doi: 10.1038/s41581-022-00561-4. Epub 2022 Apr 11. Nat Rev Nephrol. 2022. PMID: 35411063 Review.
-
Molecular basis for epithelial morphogenesis and ion transport in the Malpighian tubule.Curr Opin Insect Sci. 2021 Oct;47:7-11. doi: 10.1016/j.cois.2021.02.001. Epub 2021 Feb 10. Curr Opin Insect Sci. 2021. PMID: 33581351 Free PMC article. Review.
-
Updates on ion and water transport by the Malpighian tubule.Curr Opin Insect Sci. 2021 Oct;47:31-37. doi: 10.1016/j.cois.2021.02.018. Epub 2021 Mar 8. Curr Opin Insect Sci. 2021. PMID: 33705976 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

