Transmission of tauopathy strains is independent of their isoform composition
- PMID: 31911587
- PMCID: PMC6946697
- DOI: 10.1038/s41467-019-13787-x
Transmission of tauopathy strains is independent of their isoform composition
Abstract
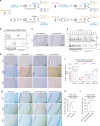
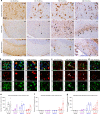
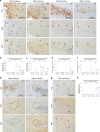
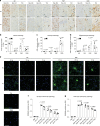
The deposition of pathological tau is a common feature in several neurodegenerative tauopathies. Although equal ratios of tau isoforms with 3 (3R) and 4 (4R) microtubule-binding repeats are expressed in the adult human brain, the pathological tau from different tauopathies have distinct isoform compositions and cell type specificities. The underlying mechanisms of tauopathies are unknown, partially due to the lack of proper models. Here, we generate a new transgenic mouse line expressing equal ratios of 3R and 4R human tau isoforms (6hTau mice). Intracerebral injections of distinct human tauopathy brain-derived tau strains into 6hTau mice recapitulate the deposition of pathological tau with distinct tau isoform compositions and cell type specificities as in human tauopathies. Moreover, through in vivo propagation of these tau strains among different mouse lines, we demonstrate that the transmission of distinct tau strains is independent of strain isoform compositions, but instead intrinsic to unique pathological conformations.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Development of a novel tau propagation mouse model endogenously expressing 3 and 4 repeat tau isoforms.Brain. 2022 Mar 29;145(1):349-361. doi: 10.1093/brain/awab289. Brain. 2022. PMID: 34515757
-
Tau mis-splicing correlates with motor impairments and striatal dysfunction in a model of tauopathy.Brain. 2021 Sep 4;144(8):2302-2309. doi: 10.1093/brain/awab130. Brain. 2021. PMID: 34059893
-
Pathological Tau Strains from Human Brains Recapitulate the Diversity of Tauopathies in Nontransgenic Mouse Brain.J Neurosci. 2017 Nov 22;37(47):11406-11423. doi: 10.1523/JNEUROSCI.1230-17.2017. Epub 2017 Oct 20. J Neurosci. 2017. PMID: 29054878 Free PMC article.
-
The Prion-Like Behavior of Assembled Tau in Transgenic Mice.Cold Spring Harb Perspect Med. 2017 Oct 3;7(10):a024372. doi: 10.1101/cshperspect.a024372. Cold Spring Harb Perspect Med. 2017. PMID: 27940600 Free PMC article. Review.
-
Cellular and pathological heterogeneity of primary tauopathies.Mol Neurodegener. 2021 Aug 23;16(1):57. doi: 10.1186/s13024-021-00476-x. Mol Neurodegener. 2021. PMID: 34425874 Free PMC article. Review.
Cited by
-
Hsp90 co-chaperones, FKBP52 and Aha1, promote tau pathogenesis in aged wild-type mice.Acta Neuropathol Commun. 2021 Apr 8;9(1):65. doi: 10.1186/s40478-021-01159-w. Acta Neuropathol Commun. 2021. PMID: 33832539 Free PMC article.
-
Uncovering specificity of endogenous TAU aggregation in a human iPSC-neuron TAU seeding model.iScience. 2021 Dec 18;25(1):103658. doi: 10.1016/j.isci.2021.103658. eCollection 2022 Jan 21. iScience. 2021. PMID: 35072001 Free PMC article.
-
Pathological and physiological functional cross-talks of α-synuclein and tau in the central nervous system.Neural Regen Res. 2024 Apr;19(4):855-862. doi: 10.4103/1673-5374.382231. Neural Regen Res. 2024. PMID: 37843221 Free PMC article. Review.
-
Understanding the Pathophysiological Actions of Tau Oligomers: A Critical Review of Current Electrophysiological Approaches.Front Mol Neurosci. 2020 Aug 20;13:155. doi: 10.3389/fnmol.2020.00155. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32973448 Free PMC article. Review.
-
Four distinct trajectories of tau deposition identified in Alzheimer's disease.Nat Med. 2021 May;27(5):871-881. doi: 10.1038/s41591-021-01309-6. Epub 2021 Apr 29. Nat Med. 2021. PMID: 33927414 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

