HIV persists throughout deep tissues with repopulation from multiple anatomical sources
- PMID: 31910162
- PMCID: PMC7108926
- DOI: 10.1172/JCI134815
HIV persists throughout deep tissues with repopulation from multiple anatomical sources
Abstract
BACKGROUNDUnderstanding HIV dynamics across the human body is important for cure efforts. This goal has been hampered by technical difficulties and the challenge of obtaining fresh tissues.METHODSThis observational study evaluated 6 individuals with HIV (n = 4 with viral suppression using antiretroviral [ART] therapy; n = 2 with rebound viremia after stopping ART), who provided serial blood samples before death and their bodies for rapid autopsy. HIV reservoirs were characterized by digital droplet PCR, single-genome amplification, and sequencing of full-length (FL) envelope HIV. Phylogeographic methods were used to reconstruct HIV spread, and generalized linear models were tested for viral factors associated with dispersal.RESULTSAcross participants, HIV DNA levels varied from approximately 0 to 659 copies/106 cells (IQR: 22.9-126.5). A total of 605 intact FL env sequences were recovered in antemortem blood cells and across 28 tissues (IQR: 5-9). Sequence analysis showed (a) the emergence of large, identical, intact HIV RNA populations in blood after cessation of therapy, which repopulated tissues throughout the body; (b) that multiple sites acted as hubs for HIV dissemination but that blood and lymphoid tissues were the main source; (c) that viral exchanges occurred within brain areas and across the blood-brain barrier; and (d) that migration was associated with low HIV divergence between sites and greater diversity at the recipient site.CONCLUSIONHIV reservoirs persisted in all deep tissues, and blood was the main source of dispersal. This may explain why eliminating HIV susceptibility in circulating T cells via bone marrow transplants allowed some individuals with HIV to experience therapy-free remission, even though deeper tissue reservoirs were not targeted.TRIAL REGISTRATIONNot applicable.FUNDINGNIH grants P01 AI31385, P30 AI036214, AI131971-01, AI120009AI036214, HD094646, AI027763, AI134295, and AI68636.
Keywords: AIDS/HIV; Infectious disease; Molecular biology.
Conflict of interest statement
Figures

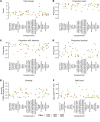
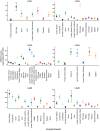


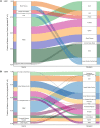
Comment in
-
The gift of a lifetime: analysis of HIV at autopsy.J Clin Invest. 2020 Apr 1;130(4):1611-1614. doi: 10.1172/JCI135905. J Clin Invest. 2020. PMID: 32091411 Free PMC article.
Similar articles
-
HIV RNA Rebound in Seminal Plasma after Antiretroviral Treatment Interruption.J Virol. 2020 Jul 16;94(15):e00415-20. doi: 10.1128/JVI.00415-20. Print 2020 Jul 16. J Virol. 2020. PMID: 32434884 Free PMC article. Clinical Trial.
-
Characterization of Intact Proviruses in Blood and Lymph Node from HIV-Infected Individuals Undergoing Analytical Treatment Interruption.J Virol. 2019 Apr 3;93(8):e01920-18. doi: 10.1128/JVI.01920-18. Print 2019 Apr 15. J Virol. 2019. PMID: 30700598 Free PMC article. Clinical Trial.
-
Size, Composition, and Evolution of HIV DNA Populations during Early Antiretroviral Therapy and Intensification with Maraviroc.J Virol. 2018 Jan 17;92(3):e01589-17. doi: 10.1128/JVI.01589-17. Print 2018 Feb 1. J Virol. 2018. PMID: 29142136 Free PMC article. Clinical Trial.
-
Residual Proviral Reservoirs: A High Risk for HIV Persistence and Driving Forces for Viral Rebound after Analytical Treatment Interruption.Viruses. 2021 Feb 21;13(2):335. doi: 10.3390/v13020335. Viruses. 2021. PMID: 33670027 Free PMC article. Review.
-
Single-molecule techniques to quantify and genetically characterise persistent HIV.Retrovirology. 2018 Jan 9;15(1):3. doi: 10.1186/s12977-017-0386-x. Retrovirology. 2018. PMID: 29316955 Free PMC article. Review.
Cited by
-
Simian-Human Immunodeficiency Virus SHIV.C.CH505 Persistence in ART-Suppressed Infant Macaques Is Characterized by Elevated SHIV RNA in the Gut and a High Abundance of Intact SHIV DNA in Naive CD4+ T Cells.J Virol. 2020 Dec 22;95(2):e01669-20. doi: 10.1128/JVI.01669-20. Print 2020 Dec 22. J Virol. 2020. PMID: 33087463 Free PMC article.
-
Emerging Single-cell Approaches to Understand HIV in the Central Nervous System.Curr HIV/AIDS Rep. 2022 Feb;19(1):113-120. doi: 10.1007/s11904-021-00586-7. Epub 2021 Nov 25. Curr HIV/AIDS Rep. 2022. PMID: 34822063 Free PMC article. Review.
-
The gift of a lifetime: analysis of HIV at autopsy.J Clin Invest. 2020 Apr 1;130(4):1611-1614. doi: 10.1172/JCI135905. J Clin Invest. 2020. PMID: 32091411 Free PMC article.
-
A Canadian Survey of Research on HIV-1 Latency-Where Are We Now and Where Are We Heading?Viruses. 2024 Feb 1;16(2):229. doi: 10.3390/v16020229. Viruses. 2024. PMID: 38400005 Free PMC article. Review.
-
Design of an optimal combination therapy with broadly neutralizing antibodies to suppress HIV-1.Elife. 2022 Jul 19;11:e76004. doi: 10.7554/eLife.76004. Elife. 2022. PMID: 35852143 Free PMC article.
References
-
- Mzingwane ML, Tiemessen CT. Mechanisms of HIV persistence in HIV reservoirs. Rev Med Virol. 2017;27(2):e1924 - PubMed

