Up-regulated fibronectin in 3D culture facilitates spreading of triple negative breast cancer cells on 2D through integrin β-5 and Src
- PMID: 31882647
- PMCID: PMC6934487
- DOI: 10.1038/s41598-019-56276-3
Up-regulated fibronectin in 3D culture facilitates spreading of triple negative breast cancer cells on 2D through integrin β-5 and Src
Abstract
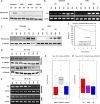
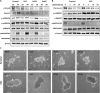
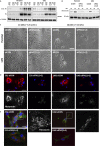
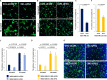
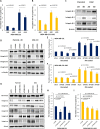
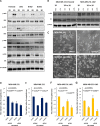
Using MDA-MB-231 cells as a model of triple negative breast cancer (TNBC) and its metastatic sub-cell lines that preferentially metastasize to lung, bone or brain, we found that the mRNA and protein levels of fibronectin (FN) are increased in MDA-MB-231 cells and its lung metastatic derivative, when cultivated in three-dimensional (3D) suspension cultures. The increase of FN expression in 3D was dependent on p38 mitogen-activated protein kinase (MAPK) because it was prevented by treatment of cells with SB203580, an inhibitor of p38MAPK. The up-regulated FN was converted into fibrils, and it enhanced cell spreading when cells cultured in 3D were transferred to two-dimensional (2D) culture. The arginine-glycine-aspartate (RGD) peptides and siRNAs targeting of integrin β-5 inhibited spreading of cells regardless of the presence of FN on 2D culture dishes. In addition, the levels of phosphorylated Src were found to be increased in 3D and the treatment of cells with SU6656, an inhibitor of Src, decreased the rate of cell spreading on FN. Collectively, these studies demonstrate that increased cellular FN in 3D suspension culture facilitates cancer cell attachment and spreading via integrin β-5 and Src, suggesting that the increased FN promotes initial attachment of cancer cells to secondary organs after circulation during metastasis.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Salvicine inactivates beta 1 integrin and inhibits adhesion of MDA-MB-435 cells to fibronectin via reactive oxygen species signaling.Mol Cancer Res. 2008 Feb;6(2):194-204. doi: 10.1158/1541-7786.MCR-07-0197. Mol Cancer Res. 2008. PMID: 18314480
-
C-terminal heparin-binding domain of fibronectin regulates integrin-mediated cell spreading but not the activation of mitogen-activated protein kinase.Biochem J. 2001 Nov 15;360(Pt 1):239-45. doi: 10.1042/0264-6021:3600239. Biochem J. 2001. PMID: 11696013 Free PMC article.
-
Binding of MMP-9-degraded fibronectin to β6 integrin promotes invasion via the FAK-Src-related Erk1/2 and PI3K/Akt/Smad-1/5/8 pathways in breast cancer.Oncol Rep. 2015 Sep;34(3):1345-52. doi: 10.3892/or.2015.4103. Epub 2015 Jul 2. Oncol Rep. 2015. PMID: 26134759
-
Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK- cell migration.EMBO J. 1998 Oct 15;17(20):5933-47. doi: 10.1093/emboj/17.20.5933. EMBO J. 1998. PMID: 9774338 Free PMC article.
-
In Situ Single-Cell Western Blot on Adherent Cell Culture.Angew Chem Int Ed Engl. 2019 Sep 23;58(39):13929-13934. doi: 10.1002/anie.201906920. Epub 2019 Aug 21. Angew Chem Int Ed Engl. 2019. PMID: 31390130 Free PMC article. Review.
Cited by
-
Spheroid Model of Mammary Tumor Cells: Epithelial-Mesenchymal Transition and Doxorubicin Response.Biology (Basel). 2024 Jun 21;13(7):463. doi: 10.3390/biology13070463. Biology (Basel). 2024. PMID: 39056658 Free PMC article.
-
Quantitative proteomic analysis of extracellular vesicle subgroups isolated by an optimized method combining polymer-based precipitation and size exclusion chromatography.J Extracell Vesicles. 2021 Apr;10(6):e12087. doi: 10.1002/jev2.12087. Epub 2021 Apr 27. J Extracell Vesicles. 2021. PMID: 33936570 Free PMC article.
-
Differential regulation of fibronectin expression and fibrillogenesis by autocrine TGF-β1 signaling in malignant and benign mammary epithelial cells.Int J Biochem Cell Biol. 2023 Dec;165:106478. doi: 10.1016/j.biocel.2023.106478. Epub 2023 Oct 21. Int J Biochem Cell Biol. 2023. PMID: 37866655 Free PMC article.
-
RGD peptide in cancer targeting: Benefits, challenges, solutions, and possible integrin-RGD interactions.Cancer Med. 2024 Jan;13(2):e6800. doi: 10.1002/cam4.6800. Cancer Med. 2024. PMID: 38349028 Free PMC article. Review.
-
Signatures of plasticity, metastasis, and immunosuppression in an atlas of human small cell lung cancer.Cancer Cell. 2021 Nov 8;39(11):1479-1496.e18. doi: 10.1016/j.ccell.2021.09.008. Epub 2021 Oct 14. Cancer Cell. 2021. PMID: 34653364 Free PMC article.
References
-
- Ekert Jason E., Johnson Kjell, Strake Brandy, Pardinas Jose, Jarantow Stephen, Perkinson Robert, Colter David C. Three-Dimensional Lung Tumor Microenvironment Modulates Therapeutic Compound Responsiveness In Vitro – Implication for Drug Development. PLoS ONE. 2014;9(3):e92248. doi: 10.1371/journal.pone.0092248. - DOI - PMC - PubMed
-
- Luca Anna C., Mersch Sabrina, Deenen René, Schmidt Stephan, Messner Isabelle, Schäfer Karl-Ludwig, Baldus Stephan E., Huckenbeck Wolfgang, Piekorz Roland P., Knoefel Wolfram T., Krieg Andreas, Stoecklein Nikolas H. Impact of the 3D Microenvironment on Phenotype, Gene Expression, and EGFR Inhibition of Colorectal Cancer Cell Lines. PLoS ONE. 2013;8(3):e59689. doi: 10.1371/journal.pone.0059689. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

