The N'-Substituted Derivatives of 5-Chloro-3-Methylisothiazole-4-Carboxylic Acid Hydrazide with Antiproliferative Activity
- PMID: 31881700
- PMCID: PMC6982951
- DOI: 10.3390/molecules25010088
The N'-Substituted Derivatives of 5-Chloro-3-Methylisothiazole-4-Carboxylic Acid Hydrazide with Antiproliferative Activity
Abstract
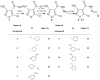
Thanks to the progress in oncology, pharmacological treatment of cancer is gaining in importance and in the near future anti-cancer chemotherapeutics are expected to be the main method of treatment for cancer diseases. What is more, the search for new anti-cancer compounds with the desired application properties is constantly underway. As a result of designed syntheses, we obtained some new N'-substituted 5-chloro-3-methylisothiazole-4-carboxylic acid hydrazide derivatives with anticancer activity. The structure of new compounds was determined by mass spectrometry (MS), elemental analysis, proton nuclear magnetic resonance spectroscopy (1H-NMR), carbon nuclear magnetic resonance spectroscopy (13C-NMR), 1H-13C NMR correlations and infrared spectroscopy (IR). Moreover, the structures of the compounds were confirmed by crystallographic examination. The antiproliferative MTT tests for 11 prepared compounds was conducted towards human biphenotypic B cell myelomonocytic leukemia MV4-11. SRB test was used to examine their potential anticancer activity towards human colon adenocarcinoma cell lines sensitive LoVo, resistant to doxorubicin LoVo/DX, breast adenocarcinoma MCF-7 and normal non-tumorigenic epithelial cell line derived from mammary gland MCF-10A. The most active compound was 5-chloro-3-methyl-N'-[(1E,2E)-(3-phenyloprop-2-en-1-ylidene]isothiazole-4-carbohydrazide, which showed the highest antiproliferative activity against all tested cell lines.
Keywords: 5-chloro-3-methylisothiazole-4-carboxylic acid hydrazide derivatives; antiproliferative activity; isothiazole.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
The 5-hydrazino-3-methylisothiazole-4-carboxylic acid, its new 5-substituted derivatives and their antiproliferative activity.Bioorg Chem. 2019 Oct;91:103082. doi: 10.1016/j.bioorg.2019.103082. Epub 2019 Jun 22. Bioorg Chem. 2019. PMID: 31351313
-
Synthesis, Characterization and Anti-Cancer Activity of Hydrazide Derivatives Incorporating a Quinoline Moiety.Molecules. 2016 Jul 14;21(7):916. doi: 10.3390/molecules21070916. Molecules. 2016. PMID: 27428941 Free PMC article.
-
Synthesis and anti-tumor evaluation of novel hydrazide and hydrazide-hydrazone derivatives.Acta Pharm. 2013 Mar;63(1):45-57. doi: 10.2478/acph-2013-0004. Acta Pharm. 2013. PMID: 23482312
-
Synthesis, structure elucidation and identification of antitumoural properties of novel fused 1,2,4-triazine aryl derivatives.Eur J Med Chem. 2008 May;43(5):1085-94. doi: 10.1016/j.ejmech.2007.07.009. Epub 2007 Jul 29. Eur J Med Chem. 2008. PMID: 17868955
-
Synthesis, Spectroscopic Analysis and Assessment of the Biological Activity of New Hydrazine and Hydrazide Derivatives of 3-Formylchromone.Molecules. 2018 Aug 17;23(8):2067. doi: 10.3390/molecules23082067. Molecules. 2018. PMID: 30126150 Free PMC article.
Cited by
-
Design and synthesis of novel ureido and thioureido conjugated hydrazone derivatives with potent anticancer activity.BMC Chem. 2022 Nov 1;16(1):81. doi: 10.1186/s13065-022-00873-3. BMC Chem. 2022. PMID: 36320042 Free PMC article.
References
-
- Yu Z., Chen Z., Sub Q., Ye S., Yuan H., Kuai M., Lv M., Tu Z., Yang X., Liue R., et al. Dual Inhibitors of RAF-MEK-ERK and PI3K-PDK1-AKT pathways: Design, Synthesis and Preliminary Anticancer Activity Studies of 3-Substituted-5-(phenylamino) indolone Derivatives. Bioorgan. Med. Chem. 2019;27:944–954. doi: 10.1016/j.bmc.2019.01.028. - DOI - PubMed
-
- El-Naggar M., Sallam H.A., Shaban S.S., Abdel-Wahab S.S., Amr A.E., Azab M.E., Nossier E.S., Al-OmaR M.A. Design, Synthesis, and Molecular Docking Study of Novel Heterocycles Incorporating 1,3,4-Thiadiazole Moiety as Potential Antimicrobial and Anticancer Agents. Molecules. 2019;24:1066. doi: 10.3390/molecules24061066. - DOI - PMC - PubMed
-
- Elsayed N.M.Y., Serya R.A.T., Tolba M.F., Ahmed M., Barakat K., Ella K.D.A.A.E., Abouzid K.A.M. Design, synthesis, biological evaluation and dynamics simulation of indazole derivatives with antiangiogenic and antiproliferative anticancer activity. Bioorgan. Chem. 2019;82:340–359. doi: 10.1016/j.bioorg.2018.10.071. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

