Characterization of Glycoproteins of Native 19kDa C-Terminal Merozoite Surface Protein-1 from Native Antigen of Plasmodium falciparum
- PMID: 31879671
- PMCID: PMC6928381
Characterization of Glycoproteins of Native 19kDa C-Terminal Merozoite Surface Protein-1 from Native Antigen of Plasmodium falciparum
Abstract
Background: Plasmodium falciparum is the protozoan parasite which causes malignant malaria of medical concern. Prime candidates for recombinant vaccine development are asexual stage antigens of P. falciparum, for example, merozoite surface proteins (MSP1 and MSP2) not given satisfactory results to date. In this study, the 19kDa C-terminal of MSP1, a vaccine candidate was purified in its native form in the ring stage, and its glycoproteins studied.
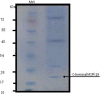
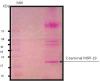
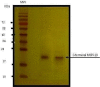
Methods: The study was carried out at the Biochemistry Department of Pasteur Institute of Iran in the years 2015-2016. Large scale culture of P. falciparum was performed in vitro with 80% ring stage parasitemia. Isopycnic ultracentrifugation with 36% sucrose and analytical SDS-PAGE on the supernatant and precipitate performed, and the 19kDa antigen was obtained by cutting it from strips of preparative SDS gels. Purified protein was concentrated and analyzed by SDS-PAGE and immunoblotting, using antibodies raised to recombinant C-terminal MSP1.
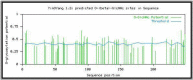
Results: The purified protein gave a single band of 19kDa antigen as shown by silver staining of SDS-PAGE and a single bond in immunoblotting. Bioinformatics also confirmed the likelihood of the presence of glycans on the antigen.
Conclusion: The presence of N and O-glycoproteins were detected by Q proteome kit. This work was done on the ring stage, and earlier workers confirmed the presence of glycoproteins on MSP1 in the other stages. This glycosylation is present in all stages, and maybe incomplete protection elicited by recombinant MSP1 antigens is due to lack of N and O-glycoproteins.
Keywords: C-terminal 19kDa; Glycoproteins; Merozoite surface protein1; Plasmodium falciparum.
Copyright© Iranian Society of Medical Entomology & Tehran University of Medical Sciences.
Figures






Similar articles
-
Human leukocyte antigen class II alleles influence levels of antibodies to the Plasmodium falciparum asexual-stage apical membrane antigen 1 but not to merozoite surface antigen 2 and merozoite surface protein 1.Infect Immun. 2004 May;72(5):2762-71. doi: 10.1128/IAI.72.5.2762-2771.2004. Infect Immun. 2004. PMID: 15102786 Free PMC article.
-
Immunity to recombinant plasmodium falciparum merozoite surface protein 1 (MSP1): protection in Aotus nancymai monkeys strongly correlates with anti-MSP1 antibody titer and in vitro parasite-inhibitory activity.Infect Immun. 2006 Aug;74(8):4573-80. doi: 10.1128/IAI.01679-05. Infect Immun. 2006. PMID: 16861644 Free PMC article.
-
Malaria parasite-inhibitory antibody epitopes on Plasmodium falciparum merozoite surface protein-1(19) mapped by TROSY NMR.Mol Biochem Parasitol. 2004 Nov;138(1):29-36. doi: 10.1016/j.molbiopara.2004.06.014. Mol Biochem Parasitol. 2004. PMID: 15500913
-
Biochemical and immunological characterization of bacterially expressed and refolded Plasmodium falciparum 42-kilodalton C-terminal merozoite surface protein 1.Infect Immun. 2003 Dec;71(12):6766-74. doi: 10.1128/IAI.71.12.6766-6774.2003. Infect Immun. 2003. PMID: 14638762 Free PMC article.
-
Dominance of conserved B-cell epitopes of the Plasmodium falciparum merozoite surface protein, MSP1, in blood-stage infections of naive Aotus monkeys.Infect Immun. 1996 May;64(5):1502-9. doi: 10.1128/iai.64.5.1502-1509.1996. Infect Immun. 1996. PMID: 8613353 Free PMC article.
Cited by
-
Post-Translational Modifications of Proteins of Malaria Parasites during the Life Cycle.Int J Mol Sci. 2024 Jun 2;25(11):6145. doi: 10.3390/ijms25116145. Int J Mol Sci. 2024. PMID: 38892332 Free PMC article. Review.
References
-
- WHO (2015) World Malaria Report. Available at: https://www.who.int/malaria/publications/world-malaria-report-2015/repor...
-
- Wright MH, Clough B, Rackham MD, Rangachari K, Brannigan JA, Grainger M, Moss DK, Bottrill AR, Heal WP, Broncel M, Serwa RA, Brady D, Mann DJ, Leatherbarrow RJ, Tewari R, Wilkinson AJ, Holder AA, Tate EW. (2014) Validation of N-myristoyltransferase as an anti-malarial drug target using an integrated chemical biology approach. Nat Chem. 6(2): 112–121. - PMC - PubMed
-
- von Itzstein M, Plebanski M, Cooke BM, Coppel RL. (2008) Hot, sweet and sticky: the glycobiology of Plasmodium falciparum. Trends Parasitol. 24(5): 210–218. - PubMed
-
- de Macedo CS, Schwarz RT, Todeschini AR, Previato JO. (2010) Mendonça-Previato L. Overlooked post-translational modifications of proteins in Plasmodium falciparum: N-and O-glycosylation-a review. Mem Inst Oswaldo Cruz. 105(8): 949–956. - PubMed
-
- Chauhan VS, Yazdani SS, Gaur D. (2010) Malaria vaccine development based on merozoite surface proteins of Plasmodium falciparum. Hum vaccin. 6(9): 10.4161.hv.6.9.12468. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
