Pasireotide treatment significantly reduces tumor volume in patients with Cushing's disease: results from a Phase 3 study
- PMID: 31875276
- PMCID: PMC7181422
- DOI: 10.1007/s11102-019-01021-2
Pasireotide treatment significantly reduces tumor volume in patients with Cushing's disease: results from a Phase 3 study
Abstract
Purpose: In the multinational, randomized, double-blind, Phase 3 B2305 study of patients with Cushing's disease (CD; ClinicalTrials.gov identifier NCT00434148), pasireotide substantially decreased urinary-free cortisol (UFC) levels, decreased mean corticotroph tumor volume, and improved clinical signs of disease. The current post hoc analysis further assesses the effects of pasireotide on corticotroph pituitary tumor volume.
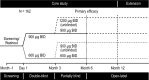
Methods: Patients enrolled in the B2305 study had persistent or recurrent CD or newly diagnosed CD but were not surgical candidates. Enrollees were randomized to receive subcutaneous pasireotide, either 600-μg or 900-μg twice daily. Tumor volume was assessed independently at months 6 and 12 by 2 blinded radiologists and compared with baseline value and UFC response.
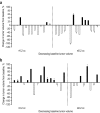
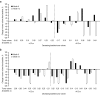
Results: Of 162 patients enrolled in the trial, 53 had measurable tumor volume data and were included in the post hoc analysis. Reductions in tumor volume were both dose and time dependent. Tumor volume reduction was more frequently observed at month 6 in the 900-μg group (75%) than in the 600-μg group (44%). Similarly, at month 12 (n = 32), tumor volume reduction was observed more frequently in the 900-µg group (89%) than in the 600-µg group (50%). Control of UFC levels was not required for reduction of tumor volume. No relationship was noted between baseline tumor size and change in tumor size.
Conclusions: Measurable decreases in pituitary tumor volume were observed in a large proportion of patients with CD and measurable tumor volume who were enrolled in the trial and treated with subcutaneous pasireotide; this decrease was not correlated with UFC control. CLINICALTRIALS.
Gov identifier: NCT00434148.
Keywords: Corticotroph tumor volume; Cushing’s disease; Pasireotide; Urinary-free cortisol.
Conflict of interest statement
AL has received funding from Novartis Pharmaceuticals Corporation to conduct clinical studies with pasireotide SC and osilodrostat (LCI699) in Cushing’s disease and served occasionally as a consultant, an advisory group member, or a speaker for Novartis, EMD Serono, and Ipsen. FG has served as a consultant, an advisory group member, an investigator, and a speaker for Novartis and Ipsen. JS has served as a lecturer, a researcher, and an advisory group member for Novartis, Ipsen and Pfizer. AK and AMP are employees of Novartis Pharma AG and hold stock options. LJ was employed by Novartis at the time of writing this manuscript but is currently employed by Bristol-Myers Squibb. RP has served as an investigator, a researcher, a consultant, and a speaker for Novartis, Viropharma, Shire, HRA Pharma, and Ipsen.
Figures
Similar articles
-
A 12-month phase 3 study of pasireotide in Cushing's disease.N Engl J Med. 2012 Mar 8;366(10):914-24. doi: 10.1056/NEJMoa1105743. N Engl J Med. 2012. PMID: 22397653 Clinical Trial.
-
Pasireotide can induce sustained decreases in urinary cortisol and provide clinical benefit in patients with Cushing's disease: results from an open-ended, open-label extension trial.Pituitary. 2015 Oct;18(5):604-12. doi: 10.1007/s11102-014-0618-1. Pituitary. 2015. PMID: 25537481 Free PMC article. Clinical Trial.
-
Salivary cortisol is a useful tool to assess the early response to pasireotide in patients with Cushing's disease.Pituitary. 2015 Feb;18(1):60-7. doi: 10.1007/s11102-014-0557-x. Pituitary. 2015. PMID: 24482099 Clinical Trial.
-
Pasireotide: a review of its use in Cushing's disease.Drugs. 2013 May;73(6):563-74. doi: 10.1007/s40265-013-0052-0. Drugs. 2013. PMID: 23605695 Review.
-
Medical treatment of Cushing's disease: somatostatin analogues and pasireotide.Neuroendocrinology. 2010;92 Suppl 1:120-4. doi: 10.1159/000314352. Epub 2010 Sep 10. Neuroendocrinology. 2010. PMID: 20829632 Review.
Cited by
-
Case Report: Opposite Tumoral and Hormonal Responses to Low-dose Pasireotide in Cushing's Disease.Endocr Metab Immune Disord Drug Targets. 2024;24(7):845-849. doi: 10.2174/0118715303260160231020070423. Endocr Metab Immune Disord Drug Targets. 2024. PMID: 37937561
-
Corticotroph tumor progression after bilateral adrenalectomy (Nelson's syndrome): systematic review and expert consensus recommendations.Eur J Endocrinol. 2021 Mar;184(3):P1-P16. doi: 10.1530/EJE-20-1088. Eur J Endocrinol. 2021. PMID: 33444221 Free PMC article.
-
Effectiveness of Medical Treatment of Cushing's Disease: A Systematic Review and Meta-Analysis.Front Endocrinol (Lausanne). 2021 Sep 17;12:732240. doi: 10.3389/fendo.2021.732240. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34603209 Free PMC article.
-
Perioperative Management of a Patient With Cushing Disease.J Endocr Soc. 2022 Jan 28;6(3):bvac010. doi: 10.1210/jendso/bvac010. eCollection 2022 Mar 1. J Endocr Soc. 2022. PMID: 35178493 Free PMC article.
-
Long-term efficacy and safety of subcutaneous pasireotide alone or in combination with cabergoline in Cushing's disease.Front Endocrinol (Lausanne). 2023 Oct 9;14:1165681. doi: 10.3389/fendo.2023.1165681. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37876540 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources

