Shear force sensing of epithelial Na+ channel (ENaC) relies on N-glycosylated asparagines in the palm and knuckle domains of αENaC
- PMID: 31871197
- PMCID: PMC6955349
- DOI: 10.1073/pnas.1911243117
Shear force sensing of epithelial Na+ channel (ENaC) relies on N-glycosylated asparagines in the palm and knuckle domains of αENaC
Abstract
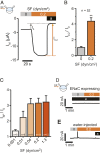
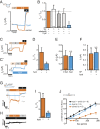
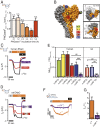
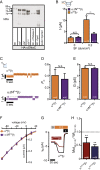
Mechanosensitive ion channels are crucial for normal cell function and facilitate physiological function, such as blood pressure regulation. So far little is known about the molecular mechanisms of how channels sense mechanical force. Canonical vertebrate epithelial Na+ channel (ENaC) formed by α-, β-, and γ-subunits is a shear force (SF) sensor and a member of the ENaC/degenerin protein family. ENaC activity in epithelial cells contributes to electrolyte/fluid-homeostasis and blood pressure regulation. Furthermore, ENaC in endothelial cells mediates vascular responsiveness to regulate blood pressure. Here, we provide evidence that ENaC's ability to mediate SF responsiveness relies on the "force-from-filament" principle involving extracellular tethers and the extracellular matrix (ECM). Two glycosylated asparagines, respectively their N-glycans localized in the palm and knuckle domains of αENaC, were identified as potential tethers. Decreased SF-induced ENaC currents were observed following removal of the ECM/glycocalyx, replacement of these glycosylated asparagines, or removal of N-glycans. Endothelial-specific overexpression of αENaC in mice induced hypertension. In contrast, expression of αENaC lacking these glycosylated asparagines blunted this effect. In summary, glycosylated asparagines in the palm and knuckle domains of αENaC are important for SF sensing. In accordance with the force-from-filament principle, they may provide a connection to the ECM that facilitates vascular responsiveness contributing to blood pressure regulation.
Keywords: N-glycosylation; epithelial Na+ channel (ENaC); extracellular tether; mechanotransduction; shear force.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures
Similar articles
-
Enhanced Shear Force Responsiveness of Epithelial Na+ Channel's (ENaC) δ Subunit Following the Insertion of N-Glycosylation Motifs Relies on the Extracellular Matrix.Int J Mol Sci. 2021 Mar 2;22(5):2500. doi: 10.3390/ijms22052500. Int J Mol Sci. 2021. PMID: 33801449 Free PMC article.
-
Epithelial Na+ channel and the glycocalyx: a sweet and salty relationship for arterial shear stress sensing.Curr Opin Nephrol Hypertens. 2022 Mar 1;31(2):142-150. doi: 10.1097/MNH.0000000000000779. Curr Opin Nephrol Hypertens. 2022. PMID: 34966089 Review.
-
Mapping allosteric linkage to channel gating by extracellular domains in the human epithelial sodium channel.J Biol Chem. 2018 Mar 9;293(10):3675-3684. doi: 10.1074/jbc.RA117.000604. Epub 2018 Jan 22. J Biol Chem. 2018. PMID: 29358325 Free PMC article.
-
Analyses of epithelial Na+ channel variants reveal that an extracellular β-ball domain critically regulates ENaC gating.J Biol Chem. 2019 Nov 8;294(45):16765-16775. doi: 10.1074/jbc.RA119.010001. Epub 2019 Sep 24. J Biol Chem. 2019. PMID: 31551351 Free PMC article.
-
ASIC and ENaC type sodium channels: conformational states and the structures of the ion selectivity filters.FEBS J. 2017 Feb;284(4):525-545. doi: 10.1111/febs.13840. Epub 2016 Sep 15. FEBS J. 2017. PMID: 27580245 Review.
Cited by
-
Force From Filaments: The Role of the Cytoskeleton and Extracellular Matrix in the Gating of Mechanosensitive Channels.Front Cell Dev Biol. 2022 May 2;10:886048. doi: 10.3389/fcell.2022.886048. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35586339 Free PMC article. Review.
-
The mechanisms of exercise improving cardiovascular function by stimulating Piezo1 and TRP ion channels: a systemic review.Mol Cell Biochem. 2025 Jan;480(1):119-137. doi: 10.1007/s11010-024-05000-5. Epub 2024 Apr 16. Mol Cell Biochem. 2025. PMID: 38625513 Review.
-
Bone marrow monocytes and macrophages from mice lacking βENaC and ASIC2 have a reduced chemotactic migration response and polarization.Physiol Rep. 2024 Jul;12(14):e16139. doi: 10.14814/phy2.16139. Physiol Rep. 2024. PMID: 39016176 Free PMC article.
-
Role of the vascular endothelial sodium channel activation in the genesis of pathologically increased cardiovascular stiffness.Cardiovasc Res. 2022 Jan 7;118(1):130-140. doi: 10.1093/cvr/cvaa326. Cardiovasc Res. 2022. PMID: 33188592 Free PMC article. Review.
-
Mechanisms of mechanotransduction and physiological roles of PIEZO channels.Nat Rev Mol Cell Biol. 2024 Nov;25(11):886-903. doi: 10.1038/s41580-024-00773-5. Epub 2024 Sep 9. Nat Rev Mol Cell Biol. 2024. PMID: 39251883 Review.
References
-
- Hamill O. P., Martinac B., Molecular basis of mechanotransduction in living cells. Physiol. Rev. 81, 685–740 (2001). - PubMed
-
- Martinac B., Adler J., Kung C., Mechanosensitive ion channels of E. coli activated by amphipaths. Nature 348, 261–263 (1990). - PubMed
-
- Katta S., Krieg M., Goodman M. B., Feeling force: Physical and physiological principles enabling sensory mechanotransduction. Annu. Rev. Cell Dev. Biol. 31, 347–371 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

