Neuroinflammation as a Factor of Neurodegenerative Disease: Thalidomide Analogs as Treatments
- PMID: 31867326
- PMCID: PMC6904283
- DOI: 10.3389/fcell.2019.00313
Neuroinflammation as a Factor of Neurodegenerative Disease: Thalidomide Analogs as Treatments
Abstract
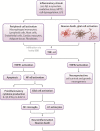
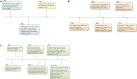
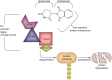
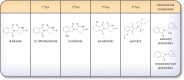
Neuroinflammation is initiated when glial cells, mainly microglia, are activated by threats to the neural environment, such as pathogen infiltration or neuronal injury. Although neuroinflammation serves to combat these threats and reinstate brain homeostasis, chronic inflammation can result in excessive cytokine production and cell death if the cause of inflammation remains. Overexpression of tumor necrosis factor-α (TNF-α), a proinflammatory cytokine with a central role in microglial activation, has been associated with neuronal excitotoxicity, synapse loss, and propagation of the inflammatory state. Thalidomide and its derivatives, termed immunomodulatory imide drugs (IMiDs), are a class of drugs that target the 3'-untranslated region (3'-UTR) of TNF-α mRNA, inhibiting TNF-α production. Due to their multi-potent effects, several IMiDs, including thalidomide, lenalidomide, and pomalidomide, have been repurposed as drug treatments for diseases such as multiple myeloma and psoriatic arthritis. Preclinical studies of currently marketed IMiDs, as well as novel IMiDs such as 3,6'-dithiothalidomide and adamantyl thalidomide derivatives, support the development of IMiDs as therapeutics for neurological disease. IMiDs have a competitive edge compared to similar anti-inflammatory drugs due to their blood-brain barrier permeability and high bioavailability, with the potential to alleviate symptoms of neurodegenerative disease and slow disease progression. In this review, we evaluate the role of neuroinflammation in neurodegenerative diseases, focusing specifically on the role of TNF-α in neuroinflammation, as well as appraise current research on the potential of IMiDs as treatments for neurological disorders.
Keywords: apremilast; immunomodulatory imide drugs (IMiDs); lenalidomide; neurodegeneration; neuroinflammation; pomalidomide; thalidomide; tumor necrosis factor-α (TNF-α).
Copyright © 2019 Jung, Tweedie, Scerba and Greig.
Figures

Similar articles
-
A New Generation of IMiDs as Treatments for Neuroinflammatory and Neurodegenerative Disorders.Biomolecules. 2023 Apr 26;13(5):747. doi: 10.3390/biom13050747. Biomolecules. 2023. PMID: 37238617 Free PMC article. Review.
-
Novel, thalidomide-like, non-cereblon binding drug tetrafluorobornylphthalimide mitigates inflammation and brain injury.J Biomed Sci. 2023 Mar 6;30(1):16. doi: 10.1186/s12929-023-00907-5. J Biomed Sci. 2023. PMID: 36872339 Free PMC article.
-
Repurposing Immunomodulatory Imide Drugs (IMiDs) in Neuropsychiatric and Neurodegenerative Disorders.Front Neurosci. 2021 Mar 29;15:656921. doi: 10.3389/fnins.2021.656921. eCollection 2021. Front Neurosci. 2021. PMID: 33854417 Free PMC article. Review.
-
Immunomodulatory properties of thalidomide analogs: pomalidomide and lenalidomide, experimental and therapeutic applications.Recent Pat Endocr Metab Immune Drug Discov. 2011 Sep;5(3):192-6. doi: 10.2174/187221411797265890. Recent Pat Endocr Metab Immune Drug Discov. 2011. PMID: 21913886 Review.
-
Activity of a Novel Anti-Inflammatory Agent F-3,6'-dithiopomalidomide as a Treatment for Traumatic Brain Injury.Biomedicines. 2022 Sep 30;10(10):2449. doi: 10.3390/biomedicines10102449. Biomedicines. 2022. PMID: 36289711 Free PMC article.
Cited by
-
Understanding the Immunologic Characteristics of Neurologic Manifestations of SARS-CoV-2 and Potential Immunological Mechanisms.Mol Neurobiol. 2020 Dec;57(12):5263-5275. doi: 10.1007/s12035-020-02094-y. Epub 2020 Sep 1. Mol Neurobiol. 2020. PMID: 32869183 Free PMC article. Review.
-
The Protective Role of Microglial PPARα in Diabetic Retinal Neurodegeneration and Neurovascular Dysfunction.Cells. 2022 Dec 1;11(23):3869. doi: 10.3390/cells11233869. Cells. 2022. PMID: 36497130 Free PMC article.
-
TNF-α-dependent neuronal necroptosis regulated in Alzheimer's disease by coordination of RIPK1-p62 complex with autophagic UVRAG.Theranostics. 2021 Sep 13;11(19):9452-9469. doi: 10.7150/thno.62376. eCollection 2021. Theranostics. 2021. PMID: 34646380 Free PMC article.
-
Neuroinflammation in Alzheimer's Disease.Biomedicines. 2021 May 7;9(5):524. doi: 10.3390/biomedicines9050524. Biomedicines. 2021. PMID: 34067173 Free PMC article. Review.
-
Current Advances of Plant-Based Vaccines for Neurodegenerative Diseases.Pharmaceutics. 2023 Feb 20;15(2):711. doi: 10.3390/pharmaceutics15020711. Pharmaceutics. 2023. PMID: 36840033 Free PMC article. Review.
References
-
- Agrawal P. (2015). Advantages and challenges in drug re-profiling. J. Pharmacovigil. 2 2–3. 10.4172/2329-6887.S2-e002 - DOI
-
- Al Saieg N., Luzar M. J. (2006). Etanercept induced multiple sclerosis and transverse myelitis. J Rheumatol. 33 1202–1204. - PubMed
-
- Alzheimer’s Association, (2019). 2019 ALZHEIMER’S DISEASE FACTS AND FIGURES Includes a Special Report on Alzheimer’s Detection in the Primary Care Setting: Connecting Patients and Physicians. Available at: https://alz.org/media/Documents/alzheimers-facts-and-figures-2019-r.pdf (accessed July 9, 2019).
Publication types
LinkOut - more resources
Full Text Sources

