Extracellular Vesicles Derived From Olfactory Ensheathing Cells Promote Peripheral Nerve Regeneration in Rats
- PMID: 31866834
- PMCID: PMC6908849
- DOI: 10.3389/fncel.2019.00548
Extracellular Vesicles Derived From Olfactory Ensheathing Cells Promote Peripheral Nerve Regeneration in Rats
Abstract
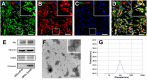
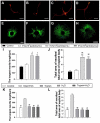
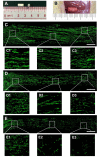
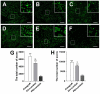
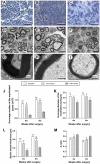
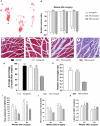
Accumulating evidence showed that extracellular vesicles (EVs) and their cargoes are important information mediators in the nervous system and have been proposed to play an important role in regulating regeneration. Moreover, many studies reported that olfactory ensheathing cells (OECs) conditioned medium is capable of promoting nerve regeneration and functional recovery. However, the role of EVs derived from OECs in axonal regeneration has not been clear. Thereby, the present study was designed to firstly isolate EVs from OECs culture supernatants, and then investigated their role in enhancing axonal regeneration after sciatic nerve injury. In vitro studies showed that OECs-EVs promoted axonal growth of dorsal root ganglion (DRG), which is dose-dependent and relies on their integrity. In vivo studies further demonstrated that nerve conduit containing OECs-EVs significantly enhanced axonal regeneration, myelination of regenerated axons and neurologically functional recovery in rats with sciatic nerve injury. In conclusion, our results, for the first time, demonstrated that OECs-EVs are capable of promoting nerve regeneration and functional recovery after peripheral nerve injuries in rats.
Keywords: extracellular vesicles; functional recovery; nerve regeneration; olfactory ensheathing cells; peripheral nerve injury.
Copyright © 2019 Xia, Gao, Li, Huang, Ma, Zhao, Yang, Huang and Luo.
Figures

Similar articles
-
A synthetic oxygen carrier-olfactory ensheathing cell composition system for the promotion of sciatic nerve regeneration.Biomaterials. 2014 Feb;35(5):1450-61. doi: 10.1016/j.biomaterials.2013.10.071. Epub 2013 Nov 15. Biomaterials. 2014. PMID: 24246645
-
Transplantation of olfactory ensheathing cells enhances peripheral nerve regeneration after microsurgical nerve repair.Brain Res. 2009 Feb 13;1254:10-7. doi: 10.1016/j.brainres.2008.11.036. Epub 2008 Nov 21. Brain Res. 2009. PMID: 19059220
-
Conditioned medium of olfactory ensheathing cells promotes the functional recovery and axonal regeneration after contusive spinal cord injury.Brain Res. 2017 Jan 1;1654(Pt A):43-54. doi: 10.1016/j.brainres.2016.10.023. Epub 2016 Oct 24. Brain Res. 2017. PMID: 27789279
-
Application of olfactory ensheathing cells in peripheral nerve injury and its complication with pathological pain.Neuroscience. 2024 Nov 12;560:120-129. doi: 10.1016/j.neuroscience.2024.09.037. Epub 2024 Sep 21. Neuroscience. 2024. PMID: 39307415 Review.
-
Peripheral nerve injuries and transplantation of olfactory ensheathing cells for axonal regeneration and remyelination: fact or fiction?Int J Mol Sci. 2012 Oct 10;13(10):12911-24. doi: 10.3390/ijms131012911. Int J Mol Sci. 2012. PMID: 23202929 Free PMC article. Review.
Cited by
-
Designing Olfactory Ensheathing Cell Transplantation Therapies: Influence of Cell Microenvironment.Cell Transplant. 2022 Jan-Dec;31:9636897221125685. doi: 10.1177/09636897221125685. Cell Transplant. 2022. PMID: 36124646 Free PMC article. Review.
-
Human Olfactory Ensheathing Cell-derived Extracellular Cesicles: miRNA Profile and Neuroprotective Effect.Curr Neurovasc Res. 2021;18(4):395-408. doi: 10.2174/1567202618666211012162111. Curr Neurovasc Res. 2021. PMID: 34645375 Free PMC article.
-
Extracellular vesicle therapy in neurological disorders.J Biomed Sci. 2024 Aug 25;31(1):85. doi: 10.1186/s12929-024-01075-w. J Biomed Sci. 2024. PMID: 39183263 Free PMC article. Review.
-
The Therapeutic Potential of Exosomes in Soft Tissue Repair and Regeneration.Int J Mol Sci. 2022 Mar 31;23(7):3869. doi: 10.3390/ijms23073869. Int J Mol Sci. 2022. PMID: 35409228 Free PMC article. Review.
-
A Novel Magnetic Responsive miR-26a@SPIONs-OECs for Spinal Cord Injury: Triggering Neural Regeneration Program and Orienting Axon Guidance in Inhibitory Astrocytic Environment.Adv Sci (Weinh). 2023 Nov;10(32):e2304487. doi: 10.1002/advs.202304487. Epub 2023 Oct 3. Adv Sci (Weinh). 2023. PMID: 37789583 Free PMC article.
References
-
- Borroto-Escuela D. O., Agnati L. F., Bechter K., Jansson A., Tarakanov A. O., Fuxe K. (2015). The role of transmitter diffusion and flow versus extracellular vesicles in volume transmission in the brain neural-glial networks. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370:20140183. 10.1098/rstb.2014.0183 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

