Thermostable β-Lactamase Mutant with Its Active Site Conjugated with Fluorescein for Efficient β-Lactam Antibiotic Detection
- PMID: 31858033
- PMCID: PMC6906784
- DOI: 10.1021/acsomega.9b02211
Thermostable β-Lactamase Mutant with Its Active Site Conjugated with Fluorescein for Efficient β-Lactam Antibiotic Detection
Abstract
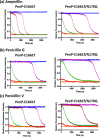
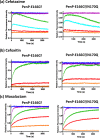
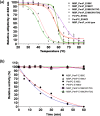
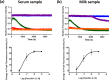
Monitoring the β-lactam antibiotic level has been an important task in food industry and clinical practice. Here, we report the development of a fluorescent PenP β-lactamase, PenP-E166Cf/N170Q, for efficient β-lactam antibiotic detection. It was constructed by covalently attaching fluorescein onto the active-site entrance of a thermostable E166Cf/N170Q mutant of a Bacillus licheniformis PenP β-lactamase. It gave a fluorescence turn-on signal toward various β-lactam antibiotics, where the fluorescence enhancement was attributed to the acyl-enzyme complex formed between PenP-E166Cf/N170Q and the β-lactam antibiotic. It demonstrated enhanced signal stability over its parental PenP-E166Cf because of the suppressed hydrolytic activity by the N170Q mutation. Compared with our previously constructed PenPC-E166Cf biosensor, PenP-E166Cf/N170Q was more thermostable and advanced in detecting β-lactams in terms of response time, signal stability, and detection limit. Positive fluorescence signals generated by E166Cf/N170Q in response to the penicillin-containing milk and mouse serum illustrated the feasibility of the biosensor for antibiotic detection in real samples. Taken together, our findings suggest the potential application of PenP-E166Cf/N170Q in biosensing β-lactam antibiotics.
Copyright © 2019 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
BADAN-conjugated β-lactamases as biosensors for β-lactam antibiotic detection.PLoS One. 2020 Oct 30;15(10):e0241594. doi: 10.1371/journal.pone.0241594. eCollection 2020. PLoS One. 2020. PMID: 33125437 Free PMC article.
-
Fluorescein-labeled beta-lactamase mutant for high-throughput screening of bacterial beta-lactamases against beta-lactam antibiotics.Anal Chem. 2005 Aug 15;77(16):5268-76. doi: 10.1021/ac0502605. Anal Chem. 2005. PMID: 16097768
-
Structural studies of the mechanism for biosensing antibiotics in a fluorescein-labeled β-lactamase.BMC Struct Biol. 2011 Mar 28;11:15. doi: 10.1186/1472-6807-11-15. BMC Struct Biol. 2011. PMID: 21443768 Free PMC article.
-
Pseudomonas aeruginosa chromosomal beta-lactamase in patients with cystic fibrosis and chronic lung infection. Mechanism of antibiotic resistance and target of the humoral immune response.APMIS Suppl. 2003;(116):1-47. APMIS Suppl. 2003. PMID: 14692154 Review.
-
Beta-lactamase inhibitors: the story so far.Curr Med Chem. 2009;16(28):3740-65. doi: 10.2174/092986709789104957. Curr Med Chem. 2009. PMID: 19747143 Review.
Cited by
-
BADAN-conjugated β-lactamases as biosensors for β-lactam antibiotic detection.PLoS One. 2020 Oct 30;15(10):e0241594. doi: 10.1371/journal.pone.0241594. eCollection 2020. PLoS One. 2020. PMID: 33125437 Free PMC article.
-
Monitoring protein conformational changes using fluorescent nanoantennas.Nat Methods. 2022 Jan;19(1):71-80. doi: 10.1038/s41592-021-01355-5. Epub 2021 Dec 30. Nat Methods. 2022. PMID: 34969985
-
Characterization of a Class A β-Lactamase from Francisella tularensis (Ftu-1) Belonging to a Unique Subclass toward Understanding AMR.ACS Bio Med Chem Au. 2023 Feb 8;3(2):174-188. doi: 10.1021/acsbiomedchemau.2c00044. eCollection 2023 Apr 19. ACS Bio Med Chem Au. 2023. PMID: 37101813 Free PMC article.
References
-
- World Health Organization . Critically Important Antimicrobials for Human Medicine, 5th revision, 2017.
LinkOut - more resources
Full Text Sources
