A web-based intervention to promote physical activity in adolescents and young adults with cystic fibrosis: protocol for a randomized controlled trial
- PMID: 31856791
- PMCID: PMC6921562
- DOI: 10.1186/s12890-019-0942-3
A web-based intervention to promote physical activity in adolescents and young adults with cystic fibrosis: protocol for a randomized controlled trial
Abstract
Background: Regular participation in physical activity by people with cystic fibrosis (CF) promotes positive clinical and health outcomes including reduced rate of decline in lung function, fewer hospitalizations and greater wellbeing. However adherence to exercise and activity programs is low, in part due to the substantial daily therapy burden for young people with CF. Strict infection control requirements limit the role of group exercise programs that are commonly used in other clinical groups. Investigation of methods to promote physical activity in this group has been limited. The Active Online Physical Activity in Cystic fibrosis Trial (ActionPACT) is an assessor-blinded, multi-centre, randomized controlled trial designed to compare the efficacy of a novel web-based program (ActivOnline) compared to usual care in promoting physical activity participation in adolescents and young adults with CF.
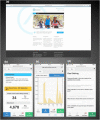
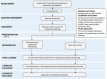
Methods: Adolescents and young adults with CF will be recruited on discharge from hospital for a respiratory exacerbation. Participants randomized to the intervention group will have access to a web-based physical activity platform for the 12-week intervention period. ActivOnline allows users to track their physical activity, set goals, and self-monitor progress. All participants in both groups will be provided with standardised information regarding general physical activity recommendations for adolescents and young adults. Outcomes will be assessed by a blinded assessor at baseline, after completion of the intervention, and at 3-months followup. Healthcare utilization will be assessed at 12 months from intervention completion. The primary outcome is change in moderate-to-vigorous physical activity participation measured objectively by accelerometry. Secondary outcomes include aerobic fitness, health-related quality of life, anxiety and depression and sleep quality.
Discussion: This trial will establish whether a web-based application can improve physical activity participation more effectively than usual care in the period following hospitalization for a respiratory exacerbation. The web-based application under investigation can be made readily and widely available to all individuals with CF, to support physical activity and exercise participation at a time and location of the user's choosing, regardless of microbiological status.
Trial registration: Clinical trial registered on July 13, 2017 with the Australian and New Zealand Clinical Trials Register at (ACTRN12617001009303).
Keywords: Application; Exercise; Goal setting; Online; Physical fitness; Telerehabilitation.
Conflict of interest statement
The authors declare they have no competing interests.
Figures
Similar articles
-
Digital technology for delivering and monitoring exercise programs for people with cystic fibrosis.Cochrane Database Syst Rev. 2023 Jun 9;6(6):CD014605. doi: 10.1002/14651858.CD014605.pub2. Cochrane Database Syst Rev. 2023. PMID: 37294546 Free PMC article. Review.
-
Web-based physical activity promotion in young people with CF: a randomised controlled trial.Thorax. 2023 Jan;78(1):16-23. doi: 10.1136/thorax-2022-218702. Epub 2022 Sep 30. Thorax. 2023. PMID: 36180067 Clinical Trial.
-
Telerehabilitation versus traditional centre-based pulmonary rehabilitation for people with chronic respiratory disease: protocol for a randomised controlled trial.BMC Pulm Med. 2018 May 15;18(1):71. doi: 10.1186/s12890-018-0646-0. BMC Pulm Med. 2018. PMID: 29764393 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Physical activity and exercise training in cystic fibrosis.Cochrane Database Syst Rev. 2022 Aug 9;8(8):CD002768. doi: 10.1002/14651858.CD002768.pub5. Cochrane Database Syst Rev. 2022. PMID: 35943025 Free PMC article. Review.
Cited by
-
Revolutionizing Care: Unleashing the Potential of Digital Health Technology in Physiotherapy Management for People With Cystic Fibrosis.JMIR Rehabil Assist Technol. 2024 Jul 15;11:e55718. doi: 10.2196/55718. JMIR Rehabil Assist Technol. 2024. PMID: 39012075 Free PMC article.
-
"Just Move It . . . Move It": A Multidisciplinary Motivational Approach to Improve Physical Activity in Children With Cystic Fibrosis.Glob Pediatr Health. 2023 Mar 6;10:2333794X221150728. doi: 10.1177/2333794X221150728. eCollection 2023. Glob Pediatr Health. 2023. PMID: 36911754 Free PMC article.
-
Sleep, Sedentary Time and Physical Activity Levels in Children with Cystic Fibrosis.Int J Environ Res Public Health. 2022 Jun 10;19(12):7133. doi: 10.3390/ijerph19127133. Int J Environ Res Public Health. 2022. PMID: 35742382 Free PMC article.
-
Digital technology for delivering and monitoring exercise programs for people with cystic fibrosis.Cochrane Database Syst Rev. 2023 Jun 9;6(6):CD014605. doi: 10.1002/14651858.CD014605.pub2. Cochrane Database Syst Rev. 2023. PMID: 37294546 Free PMC article. Review.
-
Development of a multi-wear-site, deep learning-based physical activity intensity classification algorithm using raw acceleration data.PLoS One. 2024 Mar 7;19(3):e0299295. doi: 10.1371/journal.pone.0299295. eCollection 2024. PLoS One. 2024. PMID: 38452147 Free PMC article.
References
-
- Hebestreit H, Hulzebos EHJ, Schneiderman JE, Karila C, Boas SR, Kriemler S, Dwyer T, Sahlberg M, Urquhart DS, Lands LC, et al. Cardiopulmonary exercise testing provides additional prognostic information in cystic fibrosis. Am J Respir Crit Care Med. 2019;199(8):987–995. doi: 10.1164/rccm.201806-1110OC. - DOI - PubMed

