Understanding the role of diabetes in the osteoarthritis disease and treatment process: a study protocol for the Swedish Osteoarthritis and Diabetes (SOAD) cohort
- PMID: 31852705
- PMCID: PMC6937096
- DOI: 10.1136/bmjopen-2019-032923
Understanding the role of diabetes in the osteoarthritis disease and treatment process: a study protocol for the Swedish Osteoarthritis and Diabetes (SOAD) cohort
Abstract
Introduction: Osteoarthritis (OA) is the most common form of arthritis and a leading cause of disability worldwide. Metabolic comorbidities such as type II diabetes occur with a higher rate in people with OA than in the general population. Several factors including obesity, hyperglycaemia toxicity and physical inactivity have been suggested as potential links between diabetes and OA, and have been shown to negatively impact patients' health and quality of life. However, little is known on the role of diabetes in determining the outcome of non-surgical and surgical management of OA, and at the same time, how different OA interventions may affect diabetes control. Thus, the overall aim of this project is to explore (1) the impact of diabetes on the outcome of non-surgical and surgical OA treatments and (2) the impact of non-surgical and surgical OA treatments on diabetes control.
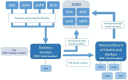
Methods and analysis: The study cohort is based on prospectively ascertained register data on a national level in Sweden. Data from OA patients who received a first-line non-surgical intervention and are registered in the National Quality Register for Better Management of Patients with Osteoarthritis will be merged with data from the Swedish Knee and Hip Arthroplasty Registers and the National Diabetes Register. Additional variables regarding patients' use of prescribed drugs, comorbidities, socioeconomic status and cause of death will be obtained through other national health and population data registers. The linkage will be performed on an individual level using unique personal identity numbers.
Ethics and dissemination: This study received ethical approval (2019-02570) from the Swedish Ethical Review Authority. Results from this cohort will be submitted to peer-reviewed scientific journals and reported at the leading national and international meetings in the field.
Keywords: cohort; exercise; general diabetes; osteoarthritis; register; surgery.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: AW-D is employed at the Swedish Knee Arthroplasty Register (SKAR). JV, A-MS, SF, EN and OR are employed by the Centre of Registers Västra Götaland, Sweden. AW-D is employed at the SKAR. LD is the co-founder and Chief Medical Officer of Joint Academy, a company that provides web-based non-surgical interventions for patients with hip and knee osteoarthritis. AA is employed by the Better Management of OsteoArthritis register.
Figures
Similar articles
-
Study protocol for an observational register-based study on health and risk factors in patients with hip and knee osteoarthritis.BMJ Open. 2018 Oct 3;8(10):e022812. doi: 10.1136/bmjopen-2018-022812. BMJ Open. 2018. PMID: 30287673 Free PMC article.
-
Socioeconomic status of patients in a Swedish national self-management program for osteoarthritis compared with the general population-a descriptive observational study.BMC Musculoskelet Disord. 2020 Jan 6;21(1):10. doi: 10.1186/s12891-019-3016-z. BMC Musculoskelet Disord. 2020. PMID: 31906904 Free PMC article.
-
Factors Associated With the Outcome of a First-Line Intervention for Patients With Hip or Knee Osteoarthritis or Both: Data From the BOA Register.Phys Ther. 2020 Sep 28;100(10):1771-1781. doi: 10.1093/ptj/pzaa113. Phys Ther. 2020. PMID: 32589713 Free PMC article.
-
OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis.Osteoarthritis Cartilage. 2019 Nov;27(11):1578-1589. doi: 10.1016/j.joca.2019.06.011. Epub 2019 Jul 3. Osteoarthritis Cartilage. 2019. PMID: 31278997 Review.
-
Results of total joint arthroplasty and joint preserving surgery in younger patients evaluated by alternative outcome measures.Dan Med J. 2014 Apr;61(4):B4836. Dan Med J. 2014. PMID: 24814600 Review.
References
-
- European Medical Agency (EMA) Guideline on clinical investigation of medicinal products used in the treatment of osteoarthritis, 2010. Available: https://www.ema.europa.eu; [Accessed 24 Sep 2019].
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
