Role of HTLV-1 orf-I encoded proteins in viral transmission and persistence
- PMID: 31852543
- PMCID: PMC6921521
- DOI: 10.1186/s12977-019-0502-1
Role of HTLV-1 orf-I encoded proteins in viral transmission and persistence
Abstract
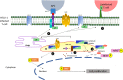
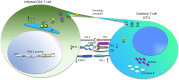
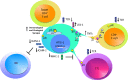
The human T cell leukemia virus type 1 (HTVL-1), first reported in 1980 by Robert Gallo's group, is the etiologic agent of both cancer and inflammatory diseases. Despite approximately 40 years of investigation, the prognosis for afflicted patients remains poor with no effective treatments. The virus persists in the infected host by evading the host immune response and inducing proliferation of infected CD4+ T-cells. Here, we will review the role that viral orf-I protein products play in altering intracellular signaling, protein expression and cell-cell communication in order to escape immune recognition and promote T-cell proliferation. We will also review studies of orf-I mutations found in infected patients and their potential impact on viral load, transmission and persistence. Finally, we will compare the orf-I gene in HTLV-1 subtypes as well as related STLV-1.
Keywords: ATLL; Adult T-cell leukemia/lymphoma; HAM/TSP; HTLV-1; HTLV-1 associated myelopathy/tropical spastic paraparesis; Immune evasion; STLV-1; orf-I; p12/p8; rex-orf-I.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Essential Role of Human T Cell Leukemia Virus Type 1 orf-I in Lethal Proliferation of CD4+ Cells in Humanized Mice.J Virol. 2019 Sep 12;93(19):e00565-19. doi: 10.1128/JVI.00565-19. Print 2019 Oct 1. J Virol. 2019. PMID: 31315992 Free PMC article.
-
Co-dependence of HTLV-1 p12 and p8 functions in virus persistence.PLoS Pathog. 2014 Nov 6;10(11):e1004454. doi: 10.1371/journal.ppat.1004454. eCollection 2014 Nov. PLoS Pathog. 2014. PMID: 25375128 Free PMC article.
-
Human T cell lymphotropic virus type I (HTLV-I)-specific CD4+ T cells: immunodominance hierarchy and preferential infection with HTLV-I.J Immunol. 2004 Feb 1;172(3):1735-43. doi: 10.4049/jimmunol.172.3.1735. J Immunol. 2004. PMID: 14734756
-
p30 protein: a critical regulator of HTLV-1 viral latency and host immunity.Retrovirology. 2019 Dec 18;16(1):42. doi: 10.1186/s12977-019-0501-2. Retrovirology. 2019. PMID: 31852501 Free PMC article. Review.
-
Silencers of HTLV-1 and HTLV-2: the pX-encoded latency-maintenance factors.Retrovirology. 2019 Sep 6;16(1):25. doi: 10.1186/s12977-019-0487-9. Retrovirology. 2019. PMID: 31492165 Free PMC article. Review.
Cited by
-
Transient Viral Activation in Human T Cell Leukemia Virus Type 1-Infected Macaques Treated With Pomalidomide.Front Med (Lausanne). 2022 May 5;9:897264. doi: 10.3389/fmed.2022.897264. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35602479 Free PMC article.
-
Immunopathogenesis and Cellular Interactions in Human T-Cell Leukemia Virus Type 1 Associated Myelopathy/Tropical Spastic Paraparesis.Front Microbiol. 2020 Dec 22;11:614940. doi: 10.3389/fmicb.2020.614940. eCollection 2020. Front Microbiol. 2020. PMID: 33414779 Free PMC article. Review.
-
Geographic distribution, clinical epidemiology and genetic diversity of the human oncogenic retrovirus HTLV-1 in Africa, the world's largest endemic area.Front Immunol. 2023 Feb 3;14:1043600. doi: 10.3389/fimmu.2023.1043600. eCollection 2023. Front Immunol. 2023. PMID: 36817417 Free PMC article. Review.
-
The role of IFN-γ production during retroviral infections: an important cytokine involved in chronic inflammation and pathogenesis.Rev Inst Med Trop Sao Paulo. 2022 Sep 30;64:e64. doi: 10.1590/S1678-9946202264064. eCollection 2022. Rev Inst Med Trop Sao Paulo. 2022. PMID: 36197425 Free PMC article. Review.
-
Epigenomic regulation of human T-cell leukemia virus by chromatin-insulator CTCF.PLoS Pathog. 2021 May 21;17(5):e1009577. doi: 10.1371/journal.ppat.1009577. eCollection 2021 May. PLoS Pathog. 2021. PMID: 34019588 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

