Antibodies to watch in 2020
- PMID: 31847708
- PMCID: PMC6973335
- DOI: 10.1080/19420862.2019.1703531
Antibodies to watch in 2020
Abstract
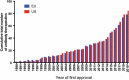
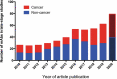
This 2020 installment of the annual 'Antibodies to Watch' series documents the antibody therapeutics approved in 2019 and in regulatory review in the United States or European Union, as well as those in late-stage clinical studies, as of November 2019*. At this time, a total of 5 novel antibody therapeutics (romosozumab, risankizumab, polatuzumab vedotin, brolucizumab, and crizanlizumab) had been granted a first approval in either the US or EU, and marketing applications for 13 novel antibody therapeutics (eptinezumab, teprotumumab, enfortumab vedotin, isatuximab, [fam-]trastuzumab deruxtecan, inebilizumab, leronlimab, sacituzumab govitecan, satralizumab, narsoplimab, tafasitamab, REGNEB3 and naxituximab) were undergoing review in these regions, which represent the major markets for antibody therapeutics. Also as of November 2019, 79 novel antibodies were undergoing evaluation in late-stage clinical studies. Of the 79 antibodies, 39 were undergoing evaluation in late-stage studies for non-cancer indications, with 2 of these (ublituximab, pamrevlumab) also in late-stage studies for cancer indications. Companies developing 7 (tanezumab, aducanumab, evinacumab, etrolizumab, sutimlimab, anifrolumab, and teplizumab) of the 39 drugs have indicated that they may submit a marketing application in either the US or EU in 2020. Of the 79 antibodies in late-stage studies, 40 were undergoing evaluation as treatments for cancer, and potentially 9 of these (belantamab mafodotin, oportuzumab monatox, margetuximab, dostarlimab, spartalizumab, 131I-omburtamab, loncastuximab tesirine, balstilimab, and zalifrelimab) may enter regulatory review in late 2019 or in 2020. Overall, the biopharmaceutical industry's clinical pipeline of antibody therapeutics is robust, and should provide a continuous supply of innovative products for patients in the future. *Note on key updates through December 18, 2019: 1) the US Food and Drug Administration granted accelerated approval to enfortumab vedotin-ejfv (Padcev) on December 18, 2019, bringing the total number of novel antibody therapeutics granted a first approval in either the US or EU during 2019 to 6; 2) the European Commission approved romosozumab on December 9, 2019; 3) the European Medicines Agency issued a positive opinion for brolucizumab; 4) Sesen Bio initiated a rolling biologics license application (BLA) on December 6, 2019; 5) GlaxoSmithKline submitted a BLA for belantamab mafodotin; and 6) the status of the Phase 3 study (NCT04128696) of GSK3359609, a humanized IgG4 anti-ICOS antibody, in patients with head and neck squamous cell carcinoma was updated to recruiting from not yet recruiting.
Keywords: Antibody therapeutics; European Medicines Agency; Food and Drug Administration; cancer; immune-mediated disorders; monoclonal antibodies.
Figures
Similar articles
-
Antibodies to watch in 2019.MAbs. 2019 Feb/Mar;11(2):219-238. doi: 10.1080/19420862.2018.1556465. Epub 2018 Dec 22. MAbs. 2019. PMID: 30516432 Free PMC article.
-
Antibodies to watch in 2018.MAbs. 2018 Feb/Mar;10(2):183-203. doi: 10.1080/19420862.2018.1415671. Epub 2018 Jan 16. MAbs. 2018. PMID: 29300693 Free PMC article.
-
Antibodies to watch in 2022.MAbs. 2022 Jan-Dec;14(1):2014296. doi: 10.1080/19420862.2021.2014296. MAbs. 2022. PMID: 35030985 Free PMC article. Review.
-
Antibodies to watch in 2017.MAbs. 2017 Feb/Mar;9(2):167-181. doi: 10.1080/19420862.2016.1269580. Epub 2016 Dec 14. MAbs. 2017. PMID: 27960628 Free PMC article.
-
Antibodies to watch in 2021.MAbs. 2021 Jan-Dec;13(1):1860476. doi: 10.1080/19420862.2020.1860476. MAbs. 2021. PMID: 33459118 Free PMC article. Review.
Cited by
-
A review of potential treatments to date in COVID-19 patients according to the stage of the disease.Curr Res Transl Med. 2020 Aug;68(3):93-104. doi: 10.1016/j.retram.2020.05.004. Epub 2020 May 30. Curr Res Transl Med. 2020. PMID: 32540367 Free PMC article. Review.
-
Mini-review: antibody therapeutics targeting G protein-coupled receptors and ion channels.Antib Ther. 2020 Dec 9;3(4):257-264. doi: 10.1093/abt/tbaa023. eCollection 2020 Dec. Antib Ther. 2020. PMID: 33912796 Free PMC article. Review.
-
Greatest Hits-Innovative Technologies for High Throughput Identification of Bispecific Antibodies.Int J Mol Sci. 2020 Sep 8;21(18):6551. doi: 10.3390/ijms21186551. Int J Mol Sci. 2020. PMID: 32911608 Free PMC article. Review.
-
Mutation of Framework Residue H71 Results in Different Antibody Paratope States in Solution.Front Immunol. 2021 Mar 2;12:630034. doi: 10.3389/fimmu.2021.630034. eCollection 2021. Front Immunol. 2021. PMID: 33737932 Free PMC article.
-
Tackling SARS-CoV-2: proposed targets and repurposed drugs.Future Med Chem. 2020 Sep;12(17):1579-1601. doi: 10.4155/fmc-2020-0147. Epub 2020 Jun 22. Future Med Chem. 2020. PMID: 32564623 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
