Optimizing Manufacturing Protocols of Chimeric Antigen Receptor T Cells for Improved Anticancer Immunotherapy
- PMID: 31835562
- PMCID: PMC6940894
- DOI: 10.3390/ijms20246223
Optimizing Manufacturing Protocols of Chimeric Antigen Receptor T Cells for Improved Anticancer Immunotherapy
Abstract
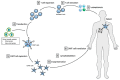
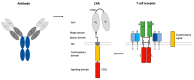
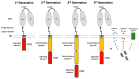
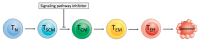
Chimeric antigen receptor (CAR) T cell therapy can achieve outstanding response rates in heavily pretreated patients with hematological malignancies. However, relapses occur and they limit the efficacy of this promising treatment approach. The cellular composition and immunophenotype of the administered CART cells play a crucial role for therapeutic success. Less differentiated CART cells are associated with improved expansion, long-term in vivo persistence, and prolonged anti-tumor control. Furthermore, the ratio between CD4+ and CD8+ T cells has an effect on the anti-tumor activity of CART cells. The composition of the final cell product is not only influenced by the CART cell construct, but also by the culturing conditions during ex vivo T cell expansion. This includes different T cell activation strategies, cytokine supplementation, and specific pathway inhibition for the differentiation blockade. The optimal production process is not yet defined. In this review, we will discuss the use of different CART cell production strategies and the molecular background for the generation of improved CART cells in detail.
Keywords: CAR; CART; CART cell production; T cell activation; T lymphocyte; adoptive cell therapy; chimeric antigen receptor; cytokines; immunotherapy.
Conflict of interest statement
Michael Schmitt received travel grants, funding for collaborative research and educational grants from Apogenix AG, Cellgene Ltd., Kite/Gilead AG, Minerva GmbH and Novartis AG. He is cofounder of TolerogenixX GmbH. Leopold Sellner is currently full-time employee of Takeda Pharma Vertrieb GmbH & Co. KG. Sophia Stock declares no conflict of interest.
Figures
Similar articles
-
Ibrutinib for improved chimeric antigen receptor T-cell production for chronic lymphocytic leukemia patients.Int J Cancer. 2021 Jan 15;148(2):419-428. doi: 10.1002/ijc.33212. Epub 2020 Jul 28. Int J Cancer. 2021. PMID: 32683672
-
Influence of Retronectin-Mediated T-Cell Activation on Expansion and Phenotype of CD19-Specific Chimeric Antigen Receptor T Cells.Hum Gene Ther. 2018 Oct;29(10):1167-1182. doi: 10.1089/hum.2017.237. Epub 2018 Sep 5. Hum Gene Ther. 2018. PMID: 30024314
-
Phase I Study of Lentiviral-Transduced Chimeric Antigen Receptor-Modified T Cells Recognizing Mesothelin in Advanced Solid Cancers.Mol Ther. 2019 Nov 6;27(11):1919-1929. doi: 10.1016/j.ymthe.2019.07.015. Epub 2019 Jul 30. Mol Ther. 2019. PMID: 31420241 Free PMC article. Clinical Trial.
-
Anti-CD123 chimeric antigen receptor T-cells (CART): an evolving treatment strategy for hematological malignancies, and a potential ace-in-the-hole against antigen-negative relapse.Leuk Lymphoma. 2018 Jul;59(7):1539-1553. doi: 10.1080/10428194.2017.1375107. Epub 2017 Sep 13. Leuk Lymphoma. 2018. PMID: 28901790 Review.
-
Immune Cell Hacking: Challenges and Clinical Approaches to Create Smarter Generations of Chimeric Antigen Receptor T Cells.Front Immunol. 2018 Jul 31;9:1717. doi: 10.3389/fimmu.2018.01717. eCollection 2018. Front Immunol. 2018. PMID: 30108584 Free PMC article. Review.
Cited by
-
In-Vivo Induced CAR-T Cell for the Potential Breakthrough to Overcome the Barriers of Current CAR-T Cell Therapy.Front Oncol. 2022 Feb 10;12:809754. doi: 10.3389/fonc.2022.809754. eCollection 2022. Front Oncol. 2022. PMID: 35223491 Free PMC article. Review.
-
Touch-free optical technologies to streamline the production of T cell therapies.Curr Opin Biomed Eng. 2023 Mar;25:100434. doi: 10.1016/j.cobme.2022.100434. Epub 2022 Dec 9. Curr Opin Biomed Eng. 2023. PMID: 36642996 Free PMC article.
-
Feasibility study of a novel preparation strategy for anti-CD7 CAR-T cells with a recombinant anti-CD7 blocking antibody.Mol Ther Oncolytics. 2022 Feb 20;24:719-728. doi: 10.1016/j.omto.2022.02.013. eCollection 2022 Mar 17. Mol Ther Oncolytics. 2022. PMID: 35317521 Free PMC article.
-
The crosstalk between immune cells and tumor pyroptosis: advancing cancer immunotherapy strategies.J Exp Clin Cancer Res. 2024 Jul 10;43(1):190. doi: 10.1186/s13046-024-03115-7. J Exp Clin Cancer Res. 2024. PMID: 38987821 Free PMC article. Review.
-
Signaling pathways in brain tumors and therapeutic interventions.Signal Transduct Target Ther. 2023 Jan 4;8(1):8. doi: 10.1038/s41392-022-01260-z. Signal Transduct Target Ther. 2023. PMID: 36596785 Free PMC article. Review.
References
-
- Rosenberg S.A., Yang J.C., Sherry R.M., Kammula U.S., Hughes M.S., Phan G.Q., Citrin D.E., Restifo N.P., Robbins P.F., Wunderlich J.R., et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. - DOI - PMC - PubMed
-
- Kunert A., Straetemans T., Govers C., Lamers C., Mathijssen R., Sleijfer S., Debets R. TCR-Engineered T Cells Meet New Challenges to Treat Solid Tumors: Choice of Antigen, T Cell Fitness, and Sensitization of Tumor Milieu. Front. Immunol. 2013;4:363. doi: 10.3389/fimmu.2013.00363. - DOI - PMC - PubMed
-
- Schubert M.L., Huckelhoven A., Hoffmann J.M., Schmitt A., Wuchter P., Sellner L., Hofmann S., Ho A.D., Dreger P., Schmitt M. Chimeric Antigen Receptor T Cell Therapy Targeting CD19-Positive Leukemia and Lymphoma in the Context of Stem Cell Transplantation. Hum. Gene Ther. 2016;27:758–771. doi: 10.1089/hum.2016.097. - DOI - PubMed
-
- Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D., et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

