Maternal betaine suppresses adrenal expression of cholesterol trafficking genes and decreases plasma corticosterone concentration in offspring pullets
- PMID: 31827786
- PMCID: PMC6862747
- DOI: 10.1186/s40104-019-0396-8
Maternal betaine suppresses adrenal expression of cholesterol trafficking genes and decreases plasma corticosterone concentration in offspring pullets
Abstract
Background: Laying hens supplemented with betaine demonstrate activated adrenal steroidogenesis and deposit higher corticosterone (CORT) in the egg yolk. Here we further investigate the effect of maternal betaine on the plasma CORT concentration and adrenal expression of steroidogenic genes in offspring pullets.
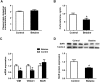
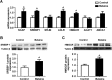
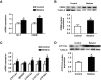
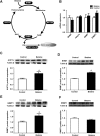
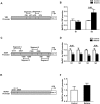
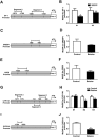
Results: Maternal betaine significantly reduced (P < 0.05) plasma CORT concentration and the adrenal expression of vimentin that is involved in trafficking cholesterol to the mitochondria for utilization in offspring pullets. Concurrently, voltage-dependent anion channel 1 and steroidogenic acute regulatory protein, the two mitochondrial proteins involved in cholesterol influx, were both down-regulated at mRNA and protein levels. However, enzymes responsible for steroid syntheses, such as cytochrome P450 family 11 subfamily A member 1 and cytochrome P450 family 21 subfamily A member 2, were significantly (P < 0.05) up-regulated at mRNA or protein levels in the adrenal gland of pullets derived from betaine-supplemented hens. Furthermore, expression of transcription factors, such as steroidogenic factor-1, sterol regulatory element-binding protein 1 and cAMP response element-binding protein, was significantly (P < 0.05) enhanced, together with their downstream target genes, such as 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, LDL receptor and sterol regulatory element-binding protein cleavage-activating protein. The promoter regions of most steroidogenic genes were significantly (P < 0.05) hypomethylated, although methyl transfer enzymes, such as AHCYL, GNMT1 and BHMT were up-regulated.
Conclusions: These results indicate that the reduced plasma CORT in betaine-supplemented offspring pullets is linked to suppressed cholesterol trafficking into the mitochondria, despite the activation of cholesterol and corticosteroid synthetic genes associated with promoter hypomethylation.
Keywords: Adrenal gland; Chicken; Cholesterol; Corticosterone; Maternal betaine; Steroidogenesis.
© The Author(s). 2019.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures
Similar articles
-
In ovo injection of betaine promotes adrenal steroidogenesis in pre-hatched chicken fetuses.Poult Sci. 2022 Jun;101(6):101871. doi: 10.1016/j.psj.2022.101871. Epub 2022 Mar 24. Poult Sci. 2022. PMID: 35487119 Free PMC article.
-
Dietary betaine supplementation increases adrenal expression of steroidogenic acute regulatory protein and yolk deposition of corticosterone in laying hens.Poult Sci. 2017 Dec 1;96(12):4389-4398. doi: 10.3382/ps/pex241. Poult Sci. 2017. PMID: 29053854
-
Betaine supplementation alleviates corticosterone-induced hepatic cholesterol accumulation through epigenetic modulation of HMGCR and CYP7A1 genes in laying hens.Poult Sci. 2024 Mar;103(3):103435. doi: 10.1016/j.psj.2024.103435. Epub 2024 Jan 6. Poult Sci. 2024. PMID: 38232620 Free PMC article.
-
Lipoprotein utilization and cholesterol synthesis by the human fetal adrenal gland.Endocr Rev. 1981 Summer;2(3):306-26. doi: 10.1210/edrv-2-3-306. Endocr Rev. 1981. PMID: 6268397 Review.
-
ACTH modulation of transcription factors responsible for steroid hydroxylase gene expression in the adrenal cortex.Microsc Res Tech. 2003 Jun 15;61(3):300-7. doi: 10.1002/jemt.10339. Microsc Res Tech. 2003. PMID: 12768545 Review.
Cited by
-
Consequence of epigenetic processes on animal health and productivity: is additional level of regulation of relevance?Anim Front. 2021 Dec 17;11(6):7-18. doi: 10.1093/af/vfab057. eCollection 2021 Dec. Anim Front. 2021. PMID: 34934525 Free PMC article. No abstract available.
-
Parental betaine supplementation promotes gosling growth with epigenetic modulation of IGF gene family in the liver.J Anim Sci. 2024 Jan 3;102:skae065. doi: 10.1093/jas/skae065. J Anim Sci. 2024. PMID: 38483185
-
In ovo injection of betaine promotes adrenal steroidogenesis in pre-hatched chicken fetuses.Poult Sci. 2022 Jun;101(6):101871. doi: 10.1016/j.psj.2022.101871. Epub 2022 Mar 24. Poult Sci. 2022. PMID: 35487119 Free PMC article.
-
Impacts of Epigenetic Processes on the Health and Productivity of Livestock.Front Genet. 2021 Feb 23;11:613636. doi: 10.3389/fgene.2020.613636. eCollection 2020. Front Genet. 2021. PMID: 33708235 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

