Milk exosomal miRNAs: potential drivers of AMPK-to-mTORC1 switching in β-cell de-differentiation of type 2 diabetes mellitus
- PMID: 31827573
- PMCID: PMC6898964
- DOI: 10.1186/s12986-019-0412-1
Milk exosomal miRNAs: potential drivers of AMPK-to-mTORC1 switching in β-cell de-differentiation of type 2 diabetes mellitus
Abstract
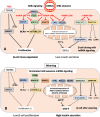
Type 2 diabetes mellitus (T2DM) steadily increases in prevalence since the 1950's, the period of widespread distribution of refrigerated pasteurized cow's milk. Whereas breastfeeding protects against the development of T2DM in later life, accumulating epidemiological evidence underlines the role of cow's milk consumption in T2DM. Recent studies in rodent models demonstrate that during the breastfeeding period pancreatic β-cells are metabolically immature and preferentially proliferate by activation of mechanistic target of rapamycin complex 1 (mTORC1) and suppression of AMP-activated protein kinase (AMPK). Weaning determines a metabolic switch of β-cells from a proliferating, immature phenotype with low insulin secretion to a differentiated mature phenotype with glucose-stimulated insulin secretion, less proliferation, reduced mTORC1- but increased AMPK activity. Translational evidence presented in this perspective implies for the first time that termination of milk miRNA transfer is the driver of this metabolic switch. miRNA-148a is a key inhibitor of AMPK and phosphatase and tensin homolog, crucial suppressors of mTORC1. β-Cells of diabetic patients return to the postnatal phenotype with high mTORC1 and low AMPK activity, explained by continuous transfer of bovine milk miRNAs to the human milk consumer. Bovine milk miRNA-148a apparently promotes β-cell de-differentiation to the immature mTORC1-high/AMPK-low phenotype with functional impairments in insulin secretion, increased mTORC1-driven endoplasmic reticulum stress, reduced autophagy and early β-cell apoptosis. In contrast to pasteurized cow's milk, milk's miRNAs are inactivated by bacterial fermentation, boiling and ultra-heat treatment and are missing in current infant formula. Persistent milk miRNA signaling adds a new perspective to the pathogenesis of T2DM and explains the protective role of breastfeeding but the diabetogenic effect of continued milk miRNA signaling by persistent consumption of pasteurized cow's milk.
Keywords: AMP-activated protein kinase; Beta-cell de-differentiation; Beta-cell metabolic switch; Diabetes mellitus type 2; Estrogen-related receptor gamma; Exosome; Mechanistic target of rapamycin complex 1; Pasteurized milk; Weaning; miRNA-148a.
© The Author(s). 2019.
Conflict of interest statement
Competing interestsThe author declares that he has no competing interests.
Figures

Similar articles
-
Lifetime Impact of Cow's Milk on Overactivation of mTORC1: From Fetal to Childhood Overgrowth, Acne, Diabetes, Cancers, and Neurodegeneration.Biomolecules. 2021 Mar 9;11(3):404. doi: 10.3390/biom11030404. Biomolecules. 2021. PMID: 33803410 Free PMC article. Review.
-
The pathogenic role of persistent milk signaling in mTORC1- and milk-microRNA-driven type 2 diabetes mellitus.Curr Diabetes Rev. 2015;11(1):46-62. doi: 10.2174/1573399811666150114100653. Curr Diabetes Rev. 2015. PMID: 25587719 Free PMC article. Review.
-
Milk Exosomal microRNAs: Postnatal Promoters of β Cell Proliferation but Potential Inducers of β Cell De-Differentiation in Adult Life.Int J Mol Sci. 2022 Sep 29;23(19):11503. doi: 10.3390/ijms231911503. Int J Mol Sci. 2022. PMID: 36232796 Free PMC article. Review.
-
Milk's Role as an Epigenetic Regulator in Health and Disease.Diseases. 2017 Mar 15;5(1):12. doi: 10.3390/diseases5010012. Diseases. 2017. PMID: 28933365 Free PMC article. Review.
-
Potential Pathogenic Impact of Cow's Milk Consumption and Bovine Milk-Derived Exosomal MicroRNAs in Diffuse Large B-Cell Lymphoma.Int J Mol Sci. 2023 Mar 23;24(7):6102. doi: 10.3390/ijms24076102. Int J Mol Sci. 2023. PMID: 37047075 Free PMC article. Review.
Cited by
-
Human Breast Milk Exosomes: Affecting Factors, Their Possible Health Outcomes, and Future Directions in Dietetics.Nutrients. 2024 Oct 17;16(20):3519. doi: 10.3390/nu16203519. Nutrients. 2024. PMID: 39458514 Free PMC article. Review.
-
Circulating microRNAs in Breast Milk and Their Potential Impact on the Infant.Nutrients. 2020 Oct 8;12(10):3066. doi: 10.3390/nu12103066. Nutrients. 2020. PMID: 33049923 Free PMC article. Review.
-
Lifetime Impact of Cow's Milk on Overactivation of mTORC1: From Fetal to Childhood Overgrowth, Acne, Diabetes, Cancers, and Neurodegeneration.Biomolecules. 2021 Mar 9;11(3):404. doi: 10.3390/biom11030404. Biomolecules. 2021. PMID: 33803410 Free PMC article. Review.
-
Exosome-Derived MicroRNAs of Human Milk and Their Effects on Infant Health and Development.Biomolecules. 2021 Jun 7;11(6):851. doi: 10.3390/biom11060851. Biomolecules. 2021. PMID: 34200323 Free PMC article. Review.
-
Dietary Epigenetic Modulators: Unravelling the Still-Controversial Benefits of miRNAs in Nutrition and Disease.Nutrients. 2024 Jan 3;16(1):160. doi: 10.3390/nu16010160. Nutrients. 2024. PMID: 38201989 Free PMC article. Review.
References
-
- https://www.statista.com/statistics/448642/per-capita-consumption-of-mil.... Accessed 28 Sept 2019.
-
- https://milchindustrie.de/wpcontent/uploads/2018/11/ProkopfDeutschland_M.... Accessed 28 Sept 2019.
LinkOut - more resources
Full Text Sources
Research Materials

