A novel CDK9 inhibitor increases the efficacy of venetoclax (ABT-199) in multiple models of hematologic malignancies
- PMID: 31827241
- PMCID: PMC7266741
- DOI: 10.1038/s41375-019-0652-0
A novel CDK9 inhibitor increases the efficacy of venetoclax (ABT-199) in multiple models of hematologic malignancies
Abstract
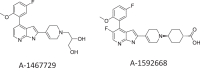
MCL-1 is one of the most frequently amplified genes in cancer, facilitating tumor initiation and maintenance and enabling resistance to anti-tumorigenic agents including the BCL-2 selective inhibitor venetoclax. The expression of MCL-1 is maintained via P-TEFb-mediated transcription, where the kinase CDK9 is a critical component. Consequently, we developed a series of potent small-molecule inhibitors of CDK9, exemplified by the orally active A-1592668, with CDK selectivity profiles that are distinct from related molecules that have been extensively studied clinically. Short-term treatment with A-1592668 rapidly downregulates RNA pol-II (Ser 2) phosphorylation resulting in the loss of MCL-1 protein and apoptosis in MCL-1-dependent hematologic tumor cell lines. This cell death could be attenuated by either inhibiting caspases or overexpressing BCL-2 protein. Synergistic cell killing was also observed between A-1592668 or the related analog A-1467729, and venetoclax in a number of hematologic cell lines and primary NHL patient samples. Importantly, the CDK9 inhibitor plus venetoclax combination was well tolerated in vivo and demonstrated efficacy superior to either agent alone in mouse models of lymphoma and AML. These data indicate that CDK9 inhibitors could be highly efficacious in tumors that depend on MCL-1 for survival or when used in combination with venetoclax in malignancies dependent on MCL-1 and BCL-2.
Conflict of interest statement
The design, study conduct, and financial support for this research were provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of this publication. DCP, SJ, JX, JC, YT, HZ, MS, SKT, RFC, TDP, DHA, JDL, and AJS are employees of AbbVie Inc. and are stock holders. GPG: Advisory board Takeda; speaker’s honoraria from Roche; research support from Amgen, Merck and AbbVie Inc.JS: Advisory boards, Celgene, Novartis, and BMS; speaker’s honoraria from Novartis, BMS, Roche, Amgen, and Takeda; research support from Amgen and Astex. RWJ: Research support from Abbvie Inc., Astra-Zeneca, BMS, Roche, and MecRX. MK: Advisory boards, F. Hoffman La-Roche and AbbVie; honoraria from Amgen, AbbVie, and Genentech; research funding from AbbVie, Genentech, Eli Lilly, Cellectis, Calithera, Stemline, Threshold, and Flexus; Reata Pharmaceuticals stock holder. EDH: Advisory board honoraria from Seattle Genetics and Celgene; research support from AbbVie Inc. and Eli Lilly. QZ, XZ, and JRD have nothing to disclose.
Figures




Similar articles
-
Flavopiridol enhances ABT-199 sensitivity in unfavourable-risk multiple myeloma cells in vitro and in vivo.Br J Cancer. 2018 Feb 6;118(3):388-397. doi: 10.1038/bjc.2017.432. Epub 2017 Dec 14. Br J Cancer. 2018. PMID: 29241222 Free PMC article.
-
A novel kinase inhibitor, LZT-106, downregulates Mcl-1 and sensitizes colorectal cancer cells to BH3 mimetic ABT-199 by targeting CDK9 and GSK-3β signaling.Cancer Lett. 2021 Feb 1;498:31-41. doi: 10.1016/j.canlet.2020.10.001. Epub 2020 Oct 28. Cancer Lett. 2021. PMID: 33129955
-
Targeting CDK9 by wogonin and related natural flavones potentiates the anti-cancer efficacy of the Bcl-2 family inhibitor ABT-263.Int J Cancer. 2015 Feb 1;136(3):688-98. doi: 10.1002/ijc.29009. Epub 2014 Jun 13. Int J Cancer. 2015. PMID: 24895203
-
Current and emerging therapies for patients with acute myeloid leukemia: a focus on MCL-1 and the CDK9 pathway.Am J Manag Care. 2018 Aug;24(16 Suppl):S356-S365. Am J Manag Care. 2018. PMID: 30132679 Review.
-
CDK9 inhibitors in multiple myeloma: a review of progress and perspectives.Med Oncol. 2022 Jan 29;39(4):39. doi: 10.1007/s12032-021-01636-1. Med Oncol. 2022. PMID: 35092513 Free PMC article. Review.
Cited by
-
Physiological and pathological roles of the transcriptional kinases CDK12 and CDK13 in the central nervous system.Cell Death Differ. 2024 Nov 12. doi: 10.1038/s41418-024-01413-3. Online ahead of print. Cell Death Differ. 2024. PMID: 39533070 Review.
-
Interrogation of novel CDK2/9 inhibitor fadraciclib (CYC065) as a potential therapeutic approach for AML.Cell Death Discov. 2021 Jun 10;7(1):137. doi: 10.1038/s41420-021-00496-y. Cell Death Discov. 2021. PMID: 34112754 Free PMC article.
-
Mechanisms of myeloid leukemogenesis: Current perspectives and therapeutic objectives.Blood Rev. 2023 Jan;57:100996. doi: 10.1016/j.blre.2022.100996. Epub 2022 Aug 2. Blood Rev. 2023. PMID: 35989139 Free PMC article. Review.
-
Oncogenic dysregulation of pre-mRNA processing by protein kinases: challenges and therapeutic opportunities.FEBS J. 2021 Nov;288(21):6250-6272. doi: 10.1111/febs.16057. Epub 2021 Jun 27. FEBS J. 2021. PMID: 34092037 Free PMC article. Review.
-
MCL-1 is a clinically targetable vulnerability in breast cancer.Cell Cycle. 2022 Jul;21(14):1439-1455. doi: 10.1080/15384101.2022.2054096. Epub 2022 Mar 29. Cell Cycle. 2022. PMID: 35349392 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

