Intrathecal, Not Systemic Inflammation Is Correlated With Multiple Sclerosis Severity, Especially in Progressive Multiple Sclerosis
- PMID: 31824409
- PMCID: PMC6884093
- DOI: 10.3389/fneur.2019.01232
Intrathecal, Not Systemic Inflammation Is Correlated With Multiple Sclerosis Severity, Especially in Progressive Multiple Sclerosis
Abstract
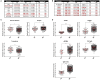
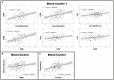
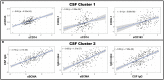
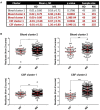
Objective: To test the hypothesis that Multiple Sclerosis (MS) patients have increased peripheral inflammation compared to healthy donors and that this systemic activation of the immune system, reflected by acute phase reactants (APRs) measured in the blood, contributes to intrathecal inflammation, which in turn contributes to the development of disability in MS. Methods: Eight serum APRs measured in a prospectively-collected cross-sectional cohort with a total of 51 healthy donors and 291 untreated MS patients were standardized and assembled into related biomarker clusters to derive global measures of systemic inflammation. The resulting APR clusters were compared between diagnostic categories and correlated to equivalently-derived cerebrospinal fluid (CSF) biomarkers of innate and adaptive immunity. Finally, correlations were calculated between biomarkers of systemic and intrathecal inflammation and MS severity measures, which predict future rates of disability progression. Results: While two blood APR clusters were elevated in MS patients, only one exhibited a weak correlation with MS severity. All CSF inflammation clusters, except CSF albumin, correlated with at least one measure of MS severity, with biomarkers of humoral adaptive immunity exhibiting the strongest correlations, especially in Progressive MS. Conclusion: Systemic inflammation does not appear to be strongly associated with intrathecal inflammation in MS. Positive correlations between markers of intrathecal inflammation, especially of humoral immunity, with MS severity measures support a pathogenic role of intrathecal (compartmentalized) inflammation in central nervous system tissue destruction, including in Progressive MS.
Keywords: T cells; acute phase reactants; adaptive immunity; cerebrospinal fluid; inflammation; innate immunity; multiple sclerosis; systemic infections.
Copyright © 2019 Milstein, Barbour, Jackson, Kosa and Bielekova.
Figures


Similar articles
-
Cerebrospinal fluid markers reveal intrathecal inflammation in progressive multiple sclerosis.Ann Neurol. 2015 Jul;78(1):3-20. doi: 10.1002/ana.24408. Epub 2015 Apr 16. Ann Neurol. 2015. PMID: 25808056 Free PMC article.
-
Cerebrospinal fluid biomarkers link toxic astrogliosis and microglial activation to multiple sclerosis severity.Mult Scler Relat Disord. 2019 Feb;28:34-43. doi: 10.1016/j.msard.2018.11.032. Epub 2018 Dec 5. Mult Scler Relat Disord. 2019. PMID: 30553167 Free PMC article. Clinical Trial.
-
Idebenone does not inhibit disability progression in primary progressive MS.Mult Scler Relat Disord. 2020 Oct;45:102434. doi: 10.1016/j.msard.2020.102434. Epub 2020 Aug 5. Mult Scler Relat Disord. 2020. PMID: 32784117 Free PMC article. Clinical Trial.
-
Exploring potential mechanisms of action of natalizumab in secondary progressive multiple sclerosis.Ther Adv Neurol Disord. 2016 Jan;9(1):31-43. doi: 10.1177/1756285615615257. Ther Adv Neurol Disord. 2016. PMID: 26788129 Free PMC article. Review.
-
Fluid biomarker and electrophysiological outcome measures for progressive MS trials.Mult Scler. 2017 Oct;23(12):1600-1613. doi: 10.1177/1352458517732844. Mult Scler. 2017. PMID: 29041870 Review.
Cited by
-
Intrathecal versus Peripheral Inflammatory Protein Profile in MS Patients at Diagnosis: A Comprehensive Investigation on Serum and CSF.Int J Mol Sci. 2023 Feb 13;24(4):3768. doi: 10.3390/ijms24043768. Int J Mol Sci. 2023. PMID: 36835179 Free PMC article.
-
Cerebrospinal Fluid IgM Levels in Association With Inflammatory Pathways in Multiple Sclerosis Patients.Front Cell Neurosci. 2020 Oct 16;14:569827. doi: 10.3389/fncel.2020.569827. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33192314 Free PMC article.
-
Effects of Natalizumab Therapy on Intrathecal Immunoglobulin G Production Indicate Targeting of Plasmablasts.Neurol Neuroimmunol Neuroinflamm. 2021 Jul 1;8(5):e1030. doi: 10.1212/NXI.0000000000001030. Print 2021 Jul. Neurol Neuroimmunol Neuroinflamm. 2021. PMID: 34210800 Free PMC article.
-
Cerebrospinal Fluid Biomarkers of Myeloid and Glial Cell Activation Are Correlated With Multiple Sclerosis Lesional Inflammatory Activity.Front Neurosci. 2021 Mar 30;15:649876. doi: 10.3389/fnins.2021.649876. eCollection 2021. Front Neurosci. 2021. PMID: 33859547 Free PMC article.
-
Immune profiling in multiple sclerosis: a single-center study of 65 cytokines, chemokines, and related molecules in cerebrospinal fluid and serum.Front Immunol. 2023 Jun 13;14:1200146. doi: 10.3389/fimmu.2023.1200146. eCollection 2023. Front Immunol. 2023. PMID: 37383229 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

