A single intra-articular injection of 2.0% non-chemically modified sodium hyaluronate vs 0.8% hylan G-F 20 in the treatment of symptomatic knee osteoarthritis: A 6-month, multicenter, randomized, controlled non-inferiority trial
- PMID: 31821355
- PMCID: PMC6903764
- DOI: 10.1371/journal.pone.0226007
A single intra-articular injection of 2.0% non-chemically modified sodium hyaluronate vs 0.8% hylan G-F 20 in the treatment of symptomatic knee osteoarthritis: A 6-month, multicenter, randomized, controlled non-inferiority trial
Abstract
Objectives: The aim of the study was to demonstrate the non-inferiority of a single intra-articular injection of 2.0% non-chemically modified sodium hyaluronate (SH) vs 0.8% hylan G-F 20 (control) in symptomatic knee osteoarthritis.
Design: This was a double-blind, randomized, controlled trial conducted in patients with painful tibiofemoral osteoarthritis (American College of Rheumatology criteria) with insufficient response or intolerance to first-line analgesics and regular non-steroidal anti-inflammatory drugs. Subjects received a single intra-articular injection of either SH or hylan G-F 20. The primary outcome was the 6-month change from baseline in the Western Ontario and McMaster Universities Osteoarthritis Index pain subscale (WOMAC A), with a pre-specified lower margin for non-inferiority of 8 mm.
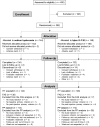
Results: Of the 292 patients randomized (SH: 144), 288 received an injection (SH: 142), 266 completed the study (SH: 134). In the Per Protocol dataset (SH: 113, control: 112), the WOMAC A change at 6 months was -34.3 mm (95% confidence interval (CI): -37.8, -30.8) and -36.2 mm (95% CI: -40.3, -32.1) for the SH and hylan G-F 20 patients, respectively (P = 0.5). The intergroup difference was -1.9 mm (95% CI: -7.3, 3.5). Results were similar in the Full Analysis Set (SH: 139, control: 141) with a difference between the groups of -2.9 mm (95% CI: -7.9, 2.2). A total of 31.3% of the injected patients reported a treatment-emergent adverse event, including injection site reactions (pain, inflammation or effusion) which occurred in 8.5% of the SH patients vs 13.0% of the hylan G-F 20 patients. No serious reactions were reported.
Conclusions: This clinical trial demonstrated the non-inferiority of a single intra-articular injection of SH vs hylan G-F 20 on the WOMAC A change from baseline at 6 months.
Conflict of interest statement
This clinical trial was sponsored by TRB Chemedica SAS, France (https://ostenil.fr/). I have read the journal's policy and the authors of this manuscript have the following competing interests: EM received personal consulting fees from TRB Chemedica, Bioiberica, Celgène, Expanscience, Genevrier, IBSA, LCA, MEDA-Mylan, Pierre Fabre Labs, and Rottapharm Biotech. BA received personal consulting fees from TRB Chemedica for his membership in the Scientific Board of this clinical trial. He also received personal consultancy fees, travel grants and honoraria for speaking or participating in congresses from Expanscience, Roche, Novartis, Bayer, Sanofi, Vertex, and Pfizer. RLD received personal consulting fees from TRB Chemedica for her membership in the Scientific Board of this clinical trial, as well as travel grants for her participation in a congress. TB received personal consulting fees from TRB Chemedica for his role in the coordination of this clinical trial. This does not alter our adherence to all PLOS ONE policies on sharing data and materials.
Figures



Similar articles
-
Intra-Articular, Single-Shot Hylan G-F 20 Hyaluronic Acid Injection Compared with Corticosteroid in Knee Osteoarthritis: A Double-Blind, Randomized Controlled Trial.J Bone Joint Surg Am. 2016 Jun 1;98(11):885-92. doi: 10.2106/JBJS.15.00544. J Bone Joint Surg Am. 2016. PMID: 27252432 Clinical Trial.
-
Evaluating the use of intra-articular injections as a treatment for painful hip osteoarthritis: a randomized, double-blind, multicenter, parallel-group study comparing a single 6-mL injection of hylan G-F 20 with saline.Osteoarthritis Cartilage. 2019 Jan;27(1):59-70. doi: 10.1016/j.joca.2018.08.018. Epub 2018 Sep 14. Osteoarthritis Cartilage. 2019. PMID: 30223023 Clinical Trial.
-
Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial.Ann Rheum Dis. 2010 Jan;69(1):113-9. doi: 10.1136/ard.2008.094623. Ann Rheum Dis. 2010. PMID: 19304567 Free PMC article. Clinical Trial.
-
Functional improvement with hylan G-F 20 in patients with knee osteoarthritis.Phys Sportsmed. 2009 Oct;37(3):38-48. doi: 10.3810/psm.2009.10.1728. Phys Sportsmed. 2009. PMID: 20048527 Review.
-
One-year efficacy and safety of single or one to three weekly injections of hylan G-F 20 for knee osteoarthritis: a systematic literature review and meta-analysis.Clin Rheumatol. 2021 Jun;40(6):2133-2142. doi: 10.1007/s10067-020-05477-7. Epub 2020 Oct 27. Clin Rheumatol. 2021. PMID: 33108530 Free PMC article. Review.
Cited by
-
Protocol for the RETHINK study: a randomised, double-blind, parallel-group, non-inferiority clinical trial comparing acetaminophen and NSAIDs for treatment of chronic pain in elderly patients with osteoarthritis of the hip and knee.BMJ Open. 2023 Feb 10;13(2):e068220. doi: 10.1136/bmjopen-2022-068220. BMJ Open. 2023. PMID: 36764707 Free PMC article.
-
Efficacy and safety of extracorporeal shock wave therapy combined with sodium hyaluronate in treatment of knee osteoarthritis: a systematic review and Meta-analysis.J Tradit Chin Med. 2024 Apr;44(2):243-250. doi: 10.19852/j.cnki.jtcm.20231226.002. J Tradit Chin Med. 2024. PMID: 38504530 Free PMC article.
-
Effectiveness of gonarthrosis treatment via intra-articular injections of linear vs. cross-linked hyaluronic acids.Jt Dis Relat Surg. 2024 Jan 1;35(1):138-145. doi: 10.52312/jdrs.2023.1403. Epub 2023 Nov 23. Jt Dis Relat Surg. 2024. PMID: 38108175 Free PMC article.
-
Intra-articular hyaluronic acid in knee osteoarthritis: clinical data for a product family (ARTHRUM), with comparative meta-analyses.Curr Ther Res Clin Exp. 2021 Sep 20;95:100637. doi: 10.1016/j.curtheres.2021.100637. eCollection 2021. Curr Ther Res Clin Exp. 2021. PMID: 34712370 Free PMC article. Review.
-
Advances in viscosupplementation and tribosupplementation for early-stage osteoarthritis therapy.Nat Rev Rheumatol. 2024 Jul;20(7):432-451. doi: 10.1038/s41584-024-01125-5. Epub 2024 Jun 10. Nat Rev Rheumatol. 2024. PMID: 38858605 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

