Predictions of hypertrophy and its regression in response to pressure overload
- PMID: 31813071
- PMCID: PMC8071348
- DOI: 10.1007/s10237-019-01271-w
Predictions of hypertrophy and its regression in response to pressure overload
Abstract
Mechanics-based cardiac growth models can now predict changes in mass, chamber size, and wall thickness in response to perturbations such as pressure overload (PO), volume overload, and myocardial infarction with a single set of growth parameters. As these models move toward clinical applications, many of the most interesting applications involve predictions of whether or how a patient's heart will reverse its growth after an intervention. In the case of PO, significant regression in wall thickness is observed both experimentally and clinically following relief of overload, for example following replacement of a stenotic aortic valve. Therefore, the objective of this work was to evaluate the ability of a published cardiac growth model that captures forward growth in multiple situations to predict growth reversal following relief of PO. Using a finite element model of a beating canine heart coupled to a circuit model of the circulation, we quantitatively matched hemodynamic data from a canine study of aortic banding followed by unbanding. Surprisingly, although the growth model correctly predicted the time course of PO-induced hypertrophy, it predicted only limited growth reversal given the measured unbanding hemodynamics, contradicting experimental and clinical observations. We were able to resolve this discrepancy only by incorporating an evolving homeostatic setpoint for the governing growth equations. Furthermore, our analysis suggests that many strain- and stress-based growth laws using the traditional volumetric growth framework will have similar difficulties capturing regression following the relief of PO unless growth setpoints are allowed to evolve.
Keywords: Finite element model; Growth; Hypertrophy; Pressure overload; Reverse growth.
Figures
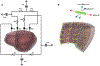
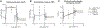
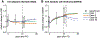

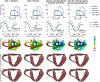

Similar articles
-
Computational models of cardiac hypertrophy.Prog Biophys Mol Biol. 2021 Jan;159:75-85. doi: 10.1016/j.pbiomolbio.2020.07.001. Epub 2020 Jul 21. Prog Biophys Mol Biol. 2021. PMID: 32702352 Free PMC article. Review.
-
Recuperative potential of cardiac muscle following relief of pressure overload hypertrophy and right ventricular failure in the cat.Circ Res. 1977 Jan;40(1):41-9. doi: 10.1161/01.res.40.1.41. Circ Res. 1977. PMID: 137086
-
Stimulus-effect relations for left ventricular growth obtained with a simple multi-scale model: the influence of hemodynamic feedback.Biomech Model Mechanobiol. 2020 Dec;19(6):2111-2126. doi: 10.1007/s10237-020-01327-2. Epub 2020 May 1. Biomech Model Mechanobiol. 2020. PMID: 32358671 Free PMC article.
-
A single strain-based growth law predicts concentric and eccentric cardiac growth during pressure and volume overload.Mech Res Commun. 2012 Jun 1;42:40-50. doi: 10.1016/j.mechrescom.2011.11.004. Epub 2011 Nov 22. Mech Res Commun. 2012. PMID: 22639476 Free PMC article.
-
[Is secondary myocardial hypertrophy a physiological or pathological adaptive mechanism?].Z Kardiol. 1982 Aug;71(8):489-96. Z Kardiol. 1982. PMID: 6215776 Review. German.
Cited by
-
Multiscale Finite Element Modeling of Left Ventricular Growth in Simulations of Valve Disease.Ann Biomed Eng. 2024 Aug;52(8):2024-2038. doi: 10.1007/s10439-024-03497-x. Epub 2024 Apr 2. Ann Biomed Eng. 2024. PMID: 38564074
-
Mechanically induced alterations in chromatin architecture guide the balance between cell plasticity and mechanical memory.Front Cell Dev Biol. 2023 Apr 18;11:1084759. doi: 10.3389/fcell.2023.1084759. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37143893 Free PMC article. Review.
-
Computational models of cardiac hypertrophy.Prog Biophys Mol Biol. 2021 Jan;159:75-85. doi: 10.1016/j.pbiomolbio.2020.07.001. Epub 2020 Jul 21. Prog Biophys Mol Biol. 2021. PMID: 32702352 Free PMC article. Review.
-
Understanding heterogeneous mechanisms of heart failure with preserved ejection fraction through cardiorenal mathematical modeling.PLoS Comput Biol. 2023 Nov 13;19(11):e1011598. doi: 10.1371/journal.pcbi.1011598. eCollection 2023 Nov. PLoS Comput Biol. 2023. PMID: 37956217 Free PMC article.
-
Mechanical stimuli for left ventricular growth during pressure overload.Exp Mech. 2021 Jan;61(1):131-146. doi: 10.1007/s11340-020-00643-z. Epub 2020 Aug 11. Exp Mech. 2021. PMID: 33746236 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

