Effect of high altitude on human placental amino acid transport
- PMID: 31804891
- PMCID: PMC6985813
- DOI: 10.1152/japplphysiol.00691.2019
Effect of high altitude on human placental amino acid transport
Abstract
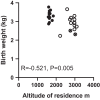
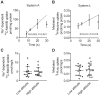
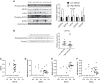
Women residing at high altitudes deliver infants of lower birth weight than at sea level. Birth weight correlates with placental system A-mediated amino acid transport capacity, and severe environmental hypoxia reduces system A activity in isolated trophoblast and the mouse placenta. However, the effect of high altitude on human placental amino acid transport remains unknown. We hypothesized that microvillous membrane (MVM) system A and system L amino acid transporter activity is lower in placentas of women living at high altitude compared with low-altitude controls. Placentas were collected at term from healthy pregnant women residing at high altitude (HA; >2,500 m; n = 14) or low altitude (LA; <1,700 m; n = 14) following planned, unlabored cesarean section. Birth weight, but not placenta weight, was 13% lower in HA pregnancies (2.88 ± 0.11 kg) compared with LA (3.30 ± 0.07 kg, P < 0.01). MVM erythropoietin receptor abundance, determined by immunoblot, was greater in HA than in LA placentas, consistent with lower placental oxygen levels at HA. However, there was no effect of altitude on MVM system A or L activity, determined by Na+-dependent [14C]methylaminoisobutyric acid uptake and [3H]leucine uptake, respectively. MVM abundance of glucose transporters (GLUTs) 1 and 4 and basal membrane GLUT4 were also similar in LA and HA placentas. Low birth weights in the neonates of women residing at high altitude are not a consequence of reduced placental amino acid transport capacity. These observations are in general agreement with studies of IUGR babies at low altitude, in which MVM system A activity is downregulated only in growth-restricted babies with significant compromise.NEW & NOTEWORTHY Babies born at high altitude are smaller than at sea level. Birth weight is dependent on growth in utero and, in turn, placental nutrient transport. We determined amino acid transport capacity in placentas collected from women resident at low and high altitude. Altitude did not affect system A amino acid transport across the syncytiotrophoblast microvillous membrane, suggesting that impaired placental amino acid transport does not contribute to reduced birth weight in this high-altitude population.
Keywords: fetal growth; glucose transporter; maternal-fetal exchange; trophoblast.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


Similar articles
-
Down-Regulation of Placental Transport of Amino Acids Precedes the Development of Intrauterine Growth Restriction in Maternal Nutrient Restricted Baboons.Biol Reprod. 2016 Nov;95(5):98. doi: 10.1095/biolreprod.116.141085. Epub 2016 Sep 7. Biol Reprod. 2016. PMID: 27605346 Free PMC article.
-
Differential levels of amino acid transporters System L and ASCT2, and the mTOR protein in placenta of preeclampsia and IUGR.BMC Pregnancy Childbirth. 2014 May 30;14:181. doi: 10.1186/1471-2393-14-181. BMC Pregnancy Childbirth. 2014. PMID: 24886642 Free PMC article.
-
Reduced placental amino acid transport in response to maternal nutrient restriction in the baboon.Am J Physiol Regul Integr Comp Physiol. 2015 Oct;309(7):R740-6. doi: 10.1152/ajpregu.00161.2015. Epub 2015 Aug 5. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 26246504 Free PMC article.
-
Placental transport of amino acids in normal and growth-restricted pregnancies.Eur J Obstet Gynecol Reprod Biol. 2003 Sep 22;110 Suppl 1:S50-4. doi: 10.1016/s0301-2115(03)00172-6. Eur J Obstet Gynecol Reprod Biol. 2003. PMID: 12965090 Review.
-
The role of trophoblast nutrient and ion transporters in the development of pregnancy complications and adult disease.Curr Vasc Pharmacol. 2009 Oct;7(4):521-33. doi: 10.2174/157016109789043982. Curr Vasc Pharmacol. 2009. PMID: 19485888 Review.
Cited by
-
Sex-biased regulatory changes in the placenta of native highlanders contribute to adaptive fetal development.Elife. 2024 Jun 13;12:RP89004. doi: 10.7554/eLife.89004. Elife. 2024. PMID: 38869160 Free PMC article.
-
Expression of nutrient transporters in placentas affected by gestational diabetes: role of leptin.Front Endocrinol (Lausanne). 2023 Jul 11;14:1172831. doi: 10.3389/fendo.2023.1172831. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37497352 Free PMC article. Review.
-
Uteroplacental nutrient flux and evidence for metabolic reprogramming during sustained hypoxemia.Physiol Rep. 2021 Sep;9(18):e15033. doi: 10.14814/phy2.15033. Physiol Rep. 2021. PMID: 34558219 Free PMC article.
-
Placental adaptations supporting fetal growth during normal and adverse gestational environments.Exp Physiol. 2023 Mar;108(3):371-397. doi: 10.1113/EP090442. Epub 2022 Dec 9. Exp Physiol. 2023. PMID: 36484327 Free PMC article. Review.
-
Cause of fetal growth restriction during high-altitude pregnancy.iScience. 2024 Apr 8;27(5):109702. doi: 10.1016/j.isci.2024.109702. eCollection 2024 May 17. iScience. 2024. PMID: 38694168 Free PMC article.
References
-
- Glazier JD, Cetin I, Perugino G, Ronzoni S, Grey AM, Mahendran D, Marconi AM, Pardi G, Sibley CP. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr Res 42: 514–519, 1997. doi:10.1203/00006450-199710000-00016. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

