Kaposi's Sarcoma-Associated Herpesvirus Viral Interleukin-6 Signaling Upregulates Integrin β3 Levels and Is Dependent on STAT3
- PMID: 31801855
- PMCID: PMC7022358
- DOI: 10.1128/JVI.01384-19
Kaposi's Sarcoma-Associated Herpesvirus Viral Interleukin-6 Signaling Upregulates Integrin β3 Levels and Is Dependent on STAT3
Abstract
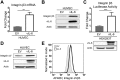
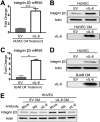
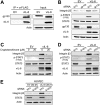
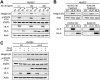
Kaposi's sarcoma-associated herpesvirus (KSHV) is the causative agent of two B-cell lymphoproliferative diseases and Kaposi's sarcoma, an endothelial-cell-driven cancer. KSHV viral interleukin-6 (vIL-6) is a viral homolog of human IL-6 (hIL-6) that is expressed in KSHV-associated malignancies. Previous studies have shown that the expression of the integrin β3 (ITGB3) subunit is induced upon KSHV infection. Here we report that KSHV vIL-6 is able to induce the expression of ITGB3 and increase surface expression of the αVβ3 integrin heterodimer. We demonstrated using small interfering RNA (siRNA) depletion and inhibitor studies that KSHV vIL-6 can increase ITGB3 by inducing STAT3 signaling. Furthermore, we found that secreted vIL-6 is capable of inducing ITGB3 in endothelial cells in a paracrine manner. Importantly, the ability to induce ITGB3 in endothelial cells seems to be specific to vIL-6, as overexpression of hIL-6 alone did not affect levels of this integrin. Our lab and others have previously shown that vIL-6 can induce angiogenesis, and we investigated whether ITGB3 was involved in this process. We found that siRNA depletion of ITGB3 in vIL-6-expressing endothelial cells resulted in a decrease in adhesion to extracellular matrix proteins. Moreover, depletion of ITGB3 hindered the ability of vIL-6 to promote angiogenesis. In conclusion, we found that vIL-6 can singularly induce ITGB3 and that this induction is dependent on vIL-6 activation of the STAT3 signaling pathway.IMPORTANCE Kaposi's sarcoma-associated herpesvirus (KSHV) is the etiological agent of three human malignancies: multicentric Castleman's disease, primary effusion lymphoma, and Kaposi's sarcoma. Kaposi's sarcoma is a highly angiogenic tumor that arises from endothelial cells. It has been previously reported that KSHV infection of endothelial cells leads to an increase of integrin αVβ3, a molecule observed to be involved in the angiogenic process of several malignancies. Our data demonstrate that the KSHV protein viral interleukin-6 (vIL-6) can induce integrin β3 in an intracellular and paracrine manner. Furthermore, we showed that this induction is necessary for vIL-6-mediated cell adhesion and angiogenesis, suggesting a potential role of integrin β3 in KSHV pathogenesis and development of Kaposi's sarcoma.
Keywords: Kaposi’s sarcoma-associated herpesvirus; integrin β3; viral IL-6.
Copyright © 2020 American Society for Microbiology.
Figures


Similar articles
-
Kaposi's Sarcoma-Associated Herpesvirus Interleukin-6 Modulates Endothelial Cell Movement by Upregulating Cellular Genes Involved in Migration.mBio. 2015 Dec 8;6(6):e01499-15. doi: 10.1128/mBio.01499-15. mBio. 2015. PMID: 26646010 Free PMC article.
-
Modulation of Kaposi's sarcoma-associated herpesvirus interleukin-6 function by hypoxia-upregulated protein 1.J Virol. 2014 Aug;88(16):9429-41. doi: 10.1128/JVI.00511-14. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920810 Free PMC article.
-
Genetic Analyses of Contributions of Viral Interleukin-6 Interactions and Signaling to Human Herpesvirus 8 Productive Replication.J Virol. 2020 Sep 15;94(19):e00909-20. doi: 10.1128/JVI.00909-20. Print 2020 Sep 15. J Virol. 2020. PMID: 32669340 Free PMC article.
-
Viral interleukin-6: role in Kaposi's sarcoma-associated herpesvirus: associated malignancies.J Interferon Cytokine Res. 2011 Nov;31(11):791-801. doi: 10.1089/jir.2011.0043. Epub 2011 Jul 18. J Interferon Cytokine Res. 2011. PMID: 21767154 Free PMC article. Review.
-
Pathological Features of Kaposi's Sarcoma-Associated Herpesvirus Infection.Adv Exp Med Biol. 2018;1045:357-376. doi: 10.1007/978-981-10-7230-7_16. Adv Exp Med Biol. 2018. PMID: 29896675 Review.
Cited by
-
Integrative Transcriptomic Analysis Reveals the Immune Mechanism for a CyHV-3-Resistant Common Carp Strain.Front Immunol. 2021 Jul 5;12:687151. doi: 10.3389/fimmu.2021.687151. eCollection 2021. Front Immunol. 2021. PMID: 34290708 Free PMC article.
-
Germinal Center Cytokines Driven Epigenetic Control of Epstein-Barr Virus Latency Gene Expression.bioRxiv [Preprint]. 2024 Jan 3:2024.01.02.573986. doi: 10.1101/2024.01.02.573986. bioRxiv. 2024. Update in: PLoS Pathog. 2024 Apr 29;20(4):e1011939. doi: 10.1371/journal.ppat.1011939 PMID: 38260430 Free PMC article. Updated. Preprint.
-
Human Gammaherpesvirus 8 Oncogenes Associated with Kaposi's Sarcoma.Int J Mol Sci. 2022 Jun 29;23(13):7203. doi: 10.3390/ijms23137203. Int J Mol Sci. 2022. PMID: 35806208 Free PMC article. Review.
-
Sporadic Kaposi Sarcoma Following a COVID-19 Vaccine: Mere Coincidence or Something More?Cureus. 2024 Feb 9;16(2):e53925. doi: 10.7759/cureus.53925. eCollection 2024 Feb. Cureus. 2024. PMID: 38465101 Free PMC article.
-
KSHV: Immune Modulation and Immunotherapy.Front Immunol. 2020 Feb 7;10:3084. doi: 10.3389/fimmu.2019.03084. eCollection 2019. Front Immunol. 2020. PMID: 32117196 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

