Restoration of Functional Full-Length Dystrophin After Intramuscular Transplantation of Foamy Virus-Transduced Myoblasts
- PMID: 31801386
- PMCID: PMC7047098
- DOI: 10.1089/hum.2019.224
Restoration of Functional Full-Length Dystrophin After Intramuscular Transplantation of Foamy Virus-Transduced Myoblasts
Abstract
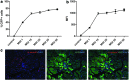
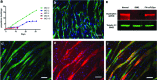
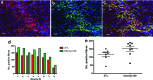
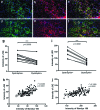
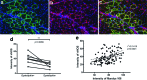
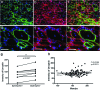
Stem cell therapy is a promising strategy to treat muscle diseases such as Duchenne muscular dystrophy (DMD). To avoid immune rejection of donor cells or donor-derived muscle, autologous cells, which have been genetically modified to express dystrophin, are preferable to cells derived from healthy donors. Restoration of full-length dystrophin (FL-dys) using viral vectors is extremely challenging, due to the limited packaging capacity of the vectors, but we have recently shown that either a foamy viral or lentiviral vector is able to package FL-dys open-reading frame and transduce myoblasts derived from a DMD patient. Differentiated myotubes derived from these transduced cells produced FL-dys. Here, we transplanted the foamy viral dystrophin-corrected DMD myoblasts intramuscularly into mdx nude mice, and showed that the transduced cells contributed to muscle regeneration, expressing FL-dys in nearly all the muscle fibers of donor origin. Furthermore, we showed that the restored FL-dys recruited members of the dystrophin-associated protein complex and neuronal nitric oxide synthase within donor-derived muscle fibers, evidence that the restored dystrophin protein is functional. Dystrophin-expressing donor-derived muscle fibers expressed lower levels of utrophin than host muscle fibers, providing additional evidence of functional improvement of donor-derived myofibers. This is the first in vivo evidence that foamy virus vector-transduced DMD myoblasts can contribute to muscle regeneration and mediate functional dystrophin restoration following their intramuscular transplantation, representing a promising therapeutic strategy for individual small muscles in DMD.
Keywords: Duchenne muscular dystrophy; codon-optimized full-length dystrophin; foamy virus; intramuscular transplantation; mdx nude mice.
Conflict of interest statement
No competing financial interests exist.
Figures
Similar articles
-
Autologous skeletal muscle derived cells expressing a novel functional dystrophin provide a potential therapy for Duchenne Muscular Dystrophy.Sci Rep. 2016 Jan 27;6:19750. doi: 10.1038/srep19750. Sci Rep. 2016. PMID: 26813695 Free PMC article.
-
Autologous Cell Therapy Approach for Duchenne Muscular Dystrophy using PiggyBac Transposons and Mesoangioblasts.Mol Ther. 2018 Apr 4;26(4):1093-1108. doi: 10.1016/j.ymthe.2018.01.021. Epub 2018 Feb 2. Mol Ther. 2018. PMID: 29503200 Free PMC article.
-
Transplantation of Dystrophin Expressing Chimeric Human Cells of Myoblast/Mesenchymal Stem Cell Origin Improves Function in Duchenne Muscular Dystrophy Model.Stem Cells Dev. 2021 Feb;30(4):190-202. doi: 10.1089/scd.2020.0161. Epub 2021 Jan 22. Stem Cells Dev. 2021. PMID: 33349121
-
[Gene therapy for muscular dystrophy].No To Hattatsu. 2004 Mar;36(2):117-23. No To Hattatsu. 2004. PMID: 15031985 Review. Japanese.
-
Chimeric Cell Therapies as a Novel Approach for Duchenne Muscular Dystrophy (DMD) and Muscle Regeneration.Biomolecules. 2024 May 13;14(5):575. doi: 10.3390/biom14050575. Biomolecules. 2024. PMID: 38785982 Free PMC article. Review.
Cited by
-
Optimized lentiviral vector to restore full-length dystrophin via a cell-mediated approach in a mouse model of Duchenne muscular dystrophy.Mol Ther Methods Clin Dev. 2022 May 2;25:491-507. doi: 10.1016/j.omtm.2022.04.015. eCollection 2022 Jun 9. Mol Ther Methods Clin Dev. 2022. PMID: 35615709 Free PMC article.
-
Transaminitis in a Three-year-old Boy with Duchenne Muscular Dystrophy.J Clin Transl Hepatol. 2020 Dec 28;8(4):474-475. doi: 10.14218/JCTH.2020.00038. Epub 2020 Oct 10. J Clin Transl Hepatol. 2020. PMID: 33447533 Free PMC article.
-
Therapeutic Strategies for Dystrophin Replacement in Duchenne Muscular Dystrophy.Front Med (Lausanne). 2022 Mar 28;9:859930. doi: 10.3389/fmed.2022.859930. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35419381 Free PMC article. Review.
-
Determinants of Retroviral Integration and Implications for Gene Therapeutic MLV-Based Vectors and for a Cure for HIV-1 Infection.Viruses. 2022 Dec 21;15(1):32. doi: 10.3390/v15010032. Viruses. 2022. PMID: 36680071 Free PMC article. Review.
-
The Donnan-dominated resting state of skeletal muscle fibers contributes to resilience and longevity in dystrophic fibers.J Gen Physiol. 2022 Jan 3;154(1):e202112914. doi: 10.1085/jgp.202112914. Epub 2021 Nov 3. J Gen Physiol. 2022. PMID: 34731883 Free PMC article.
References
-
- Mendell JR, Shilling C, Leslie ND, et al. . Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol 2012;71:304–313 - PubMed
-
- Collins CA, Olsen I, Zammit PS, et al. . Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 2005;122:289–301 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

