Sequential inactivated (IPV) and live oral (OPV) poliovirus vaccines for preventing poliomyelitis
- PMID: 31801180
- PMCID: PMC6953375
- DOI: 10.1002/14651858.CD011260.pub2
Sequential inactivated (IPV) and live oral (OPV) poliovirus vaccines for preventing poliomyelitis
Abstract
Background: Poliomyelitis mainly affects unvaccinated children under five years of age, causing irreversible paralysis or even death. The oral polio vaccine (OPV) contains live attenuated virus, which can, in rare cases, cause a paralysis known as vaccine-associated paralytic polio (VAPP), and also vaccine-derived polioviruses (VDPVs) due to acquired neurovirulence after prolonged duration of replication. The incidence of poliomyelitis caused by wild polio virus (WPV) has declined dramatically since the introduction of OPV and later the inactivated polio vaccine (IPV), however, the cases of paralysis linked to the OPV are currently more frequent than those related to the WPV. Therefore, in 2016, the World Health Organization (WHO) recommended at least one IPV dose preceding routine immunisation with OPV to reduce VAPPs and VDPVs until polio could be eradicated.
Objectives: To assess the effectiveness, safety, and immunogenicity of sequential IPV-OPV immunisation schemes compared to either OPV or IPV alone.
Search methods: In May 2019 we searched CENTRAL, MEDLINE, Embase, 14 other databases, three trials registers and reports of adverse effects on four web sites. We also searched the references of identified studies, relevant reviews and contacted authors to identify additional references.
Selection criteria: Randomised controlled trials (RCTs), quasi-RCTs, controlled before-after studies, nationwide uncontrolled before-after studies (UBAs), interrupted time series (ITS) and controlled ITS comparing sequential IPV-OPV schedules (one or more IPV doses followed by one or more OPV doses) with IPV alone, OPV alone or non-sequential IPV-OPV combinations.
Data collection and analysis: We used standard methodological procedures expected by Cochrane.
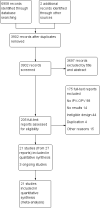
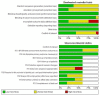
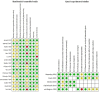
Main results: We included 21 studies: 16 RCTs involving 6407 healthy infants (age range 96 to 975 days, mean 382 days), one ITS with 28,330 infants and four nationwide studies (two ITS, two UBA). Ten RCTs were conducted in high-income countries; five in the USA, two in the UK, and one each in Chile, Israel, and Oman. The remaining six RCTs were conducted in middle-income countries; China, Bangladesh, Guatemala, India, and Thailand. We rated all included RCTs at low or unclear risk of bias for randomisation domains, most at high or unclear risk of attrition bias, and half at high or unclear risk for conflict of interests. Almost all RCTs were at low risk for the remaining domains. ITSs and UBAs were mainly considered at low risk of bias for most domains. IPV-OPV versus OPV It is uncertain if an IPV followed by OPV schedule is better than OPV alone at reducing the number of WPV cases (very low-certainty evidence); however, it may reduce VAPP cases by 54% to 100% (three nationwide studies; low-certainty evidence). There is little or no difference in vaccination coverage between IPV-OPV and OPV-only schedules (risk ratio (RR) 1.01, 95% confidence interval (CI) 0.96 to 1.06; 1 ITS study; low-certainty evidence). Similarly, there is little or no difference between the two schedule types for the number of serious adverse events (SAEs) (RR 0.88, 95% CI 0.46 to 1.70; 4 studies, 1948 participants; low-certainty evidence); or the number of people with protective humoral response P1 (moderate-certainty evidence), P2 (for the most studied schedule; two IPV doses followed by OPV; low-certainty evidence), and P3 (low-certainty evidence). Two IPV doses followed by bivalent OPV (IIbO) may reduce P2 neutralising antibodies compared to trivalent OPV (moderate-certainty evidence), but may make little or no difference to P1 or P2 neutralising antibodies following an IIO schedule or OPV alone (low-certainty evidence). Both IIO and IIbO schedules may increase P3 neutralising antibodies compared to OPV (moderate-certainty evidence). It may also lead to lower mucosal immunity given increased faecal excretion of P1 (low-certainty evidence), P2 and P3 (moderate-certainty evidence) after OPV challenge. IPV-OPV versus IPV It is uncertain if IPV-OPV is more effective than IPV alone at reducing the number of WPV cases (very low-certainty evidence). There were no data regarding VAPP cases. There is no clear evidence of a difference between IPV-OPV and OPV schedules for the number of people with protective humoral response (low- and moderate-certainty evidence). IPV-OPV schedules may increase mean titres of P1 neutralising antibodies compared to OPV alone (low- and moderate-certainty evidence), but the effect on P2 and P3 titres is not clear (very low- and moderate-certainty evidence). IPV-OPV probably reduces the number of people with P3 poliovirus faecal excretion after OPV challenge with IIO and IIOO sequences (moderate-certainty evidence), and may reduce the number with P2 (low-certainty evidence), but not with P1 (very low-certainty evidence). There may be little or no difference between the schedules in number of SAEs (RR 0.92, 95% CI 0.60 to 1.43; 2 studies, 1063 participants, low-certainty evidence). The number of persons with P2 protective humoral immunity and P2 neutralising antibodies are probably lower with most sequential schemes without P2 components (i.e. bOPV) than with trivalent OPV or IVP alone (moderate-certainty evidence). IPV (3)-OPV versus IPV (2)-OPV One study (137 participants) showed no clear evidence of a difference between three IPV doses followed by OPV and two IPV doses followed by OPV, on the number of people with P1 (RR 0.98, 95% CI 0.93 to 1.03), P2 (RR 1.00, 95% CI 0.97 to 1.03), or P3 (RR 1.01, 95% CI 0.97 to 1.05) protective humoral and intestinal immunity; all moderate-certainty evidence. This study did not report on any other outcomes.
Authors' conclusions: IPV-OPV compared to OPV may reduce VAPPs without affecting vaccination coverage, safety or humoral response, except P2 with sequential schemes without P2 components, but increase poliovirus faecal excretion after OPV challenge for some polio serotypes. Compared to IPV-only schedules, IPV-OPV may have little or no difference on SAEs, probably has little or no effect on persons with protective humoral response, may increase neutralising antibodies, and probably reduces faecal excretion after OPV challenge of certain polio serotypes. Using three IPV doses as part of a IPV-OPV schedule does not appear to be better than two IPV doses for protective humoral response. Sequential schedules during the transition from OPV to IPV-only immunisation schedules seems a reasonable option aligned with current WHO recommendations. Findings could help decision-makers to optimise polio vaccination policies, reducing inequities between countries.
Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
A preliminary version of this review was supported by a grant from the Pan American Health Organization (PAHO). The PAHO commissioned the review team to analyse the evidence for sequential inactivated (IPV) and live oral (OPV) poliovirus vaccines for the prevention of poliomyelitis, to assist their decision‐making process. Agustín Ciapponi, Ariel Bardach, Lucila Rey‐Ares, Demián Glujovsky, María Luisa Cafferata, and Silvana Cesaroni all received payments from this grant. They have no other conflicts of interest to declare.
Aikant Bhatti ‐ none known.
Disclaimer: the views herein are those of the authors and not necessarily those of the PAHO.
Figures































Update of
- doi: 10.1002/14651858.CD011260
Similar articles
-
Inactivated poliovirus vaccine given alone or in a sequential schedule with bivalent oral poliovirus vaccine in Chilean infants: a randomised, controlled, open-label, phase 4, non-inferiority study.Lancet Infect Dis. 2015 Nov;15(11):1273-82. doi: 10.1016/S1473-3099(15)00219-4. Epub 2015 Aug 26. Lancet Infect Dis. 2015. PMID: 26318714 Clinical Trial.
-
Equivalent schedules of intradermal fractional dose versus intramuscular full dose of inactivated polio vaccine for prevention of poliomyelitis.Cochrane Database Syst Rev. 2019 Dec 19;12(12):CD011780. doi: 10.1002/14651858.CD011780.pub2. Cochrane Database Syst Rev. 2019. PMID: 31858595 Free PMC article.
-
Surveillance for poliovirus vaccine adverse events, 1991 to 1998: impact of a sequential vaccination schedule of inactivated poliovirus vaccine followed by oral poliovirus vaccine.Pediatrics. 2001 May;107(5):E83. doi: 10.1542/peds.107.5.e83. Pediatrics. 2001. PMID: 11331733
-
Poliovirus vaccines. Progress toward global poliomyelitis eradication and changing routine immunization recommendations in the United States.Pediatr Clin North Am. 2000 Apr;47(2):287-308. doi: 10.1016/s0031-3955(05)70208-x. Pediatr Clin North Am. 2000. PMID: 10761505 Review.
-
Immunogenicity of New Primary Immunization Schedules With Inactivated Poliovirus Vaccine and Bivalent Oral Polio Vaccine for the Polio Endgame: A Review.Clin Infect Dis. 2018 Oct 30;67(suppl_1):S35-S41. doi: 10.1093/cid/ciy633. Clin Infect Dis. 2018. PMID: 30376081 Free PMC article. Review.
Cited by
-
Heterologous Booster Immunization Based on Inactivated SARS-CoV-2 Vaccine Enhances Humoral Immunity and Promotes BCR Repertoire Development.Vaccines (Basel). 2024 Jan 24;12(2):120. doi: 10.3390/vaccines12020120. Vaccines (Basel). 2024. PMID: 38400104 Free PMC article.
-
The Fight against Poliovirus Is Not Over.Microorganisms. 2023 May 17;11(5):1323. doi: 10.3390/microorganisms11051323. Microorganisms. 2023. PMID: 37317297 Free PMC article. Review.
-
Immuno-persistence of the different primary polio vaccine schedules and immunogenicity of the booster dose by sabin inactivated or bivalent oral poliovirus vaccine in children aged 4 years: an open-label, randomised, controlled phase 4 trial in China.Lancet Reg Health West Pac. 2023 Mar 17;34:100725. doi: 10.1016/j.lanwpc.2023.100725. eCollection 2023 May. Lancet Reg Health West Pac. 2023. PMID: 37283972 Free PMC article.
-
Comparative Study on MNVT of OPV Type I and III Reference Products in Different Periods.Diseases. 2023 Feb 8;11(1):28. doi: 10.3390/diseases11010028. Diseases. 2023. PMID: 36810542 Free PMC article.
-
The inactivation and destruction of viruses by reactive oxygen species generated through physical and cold atmospheric plasma techniques: Current status and perspectives.J Adv Res. 2023 Jan;43:59-71. doi: 10.1016/j.jare.2022.03.002. Epub 2022 Mar 9. J Adv Res. 2023. PMID: 36585115 Free PMC article. Review.
References
References to studies included in this review
Alexander 2004 {published data only}
Anand 2015 {published data only}
-
- Anand A, Zaman K, Estívariz CF, Yunus M, Gary HE, Weldon WC, et al. Early priming with inactivated poliovirus vaccine (IPV) and intradermal fractional dose IPV administered by a microneedle device: a randomized controlled trial. Vaccine 2015;33(48):6816‐22. [DOI: 10.1016/j.vaccine.2015.09.039; PUBMED: 26476367] - DOI - PMC - PubMed
Asturias 2007 {published data only}
Davis 2001 {published data only}
-
- Davis RL, Lieu TA, Mell LK, Capra AM, Zavitkovsky A, Quesenberry CP Jr, et al. Impact of the change in polio vaccination schedule on immunization coverage rates: a study in two large health maintenance organizations. Pediatrics 2001;107(4):671‐6. [DOI: 10.1542/peds.107.4.671; PUBMED: 11335742] - DOI - PubMed
Faden 1990 {published data only}
-
- Faden H. Results of a clinical study of polio vaccine: the Buffalo experience. Pediatric Infectious Disease Journal 1991;10(12):973‐5. [PUBMED: 1766725] - PubMed
-
- Faden H, Modlin JF, Thoms ML, McBean AM, Ferdon MB, Ogra PL. Comparative evaluation of immunization with live attenuated and enhanced‐potency inactivated trivalent poliovirus vaccines in childhood: systemic and local immune responses. Journal of Infectious Diseases 1990;162(6):1291‐7. [DOI: 10.1093/infdis/162.6.1291; PUBMED: 2172403] - DOI - PubMed
Halsey 1997 {published data only}
-
- Halsey NA, Blatter M, Bader G, Thoms ML, Willingham FF, O'Donovan JC, et al. Inactivated poliovirus vaccine alone or sequential inactivated and oral poliovirus vaccine in two‐, four‐ and six‐month‐old infants with combination Haemophilus influenzae type b/hepatitis B vaccine. Pediatric Infectious Disease Journal 1997;16(7):675‐9. [DOI: 10.1097/00006454-199707000-00010; PUBMED: 9239772] - DOI - PubMed
Ivanova 2018 {published data only}
-
- Ivanova OE, Eremeeva TP, Morozova NS, Shakaryan AK, Gmyl AP, Yakovenko ML, et al. Vaccine‐associated paralytic poliomyelitis in Russian Federation during the period of changes in vaccination schedule (2006‐2013 yy.) [Вакцинный паралитический полиомиелит в Российской Федерации в период изменений (2006‐2013 гг.)]. Voprosy Virusologii 2016;61(1):9‐15. [DOI: 10.18821/0507-4088-2016-61-1-9-15; PUBMED: 27145594] - DOI - PubMed
Jain 1997 {published data only}
Kapusinszky 2010 {published data only}
-
- Kapusinszky B, Molnár Z, Szomor KN, Berencsi G. Molecular characterization of poliovirus isolates from children who contracted vaccine‐associated paralytic poliomyelitis (VAPP) following administration of monovalent type 3 oral poliovirus vaccine in the 1960s in Hungary. FEMS Immunology and Medical Microbiology 2010;58(2):211‐7. [DOI: 10.1111/j.1574-695X.2009.00621.x; PUBMED: 19863665] - DOI - PubMed
Li 2016a {published data only}
-
- Li RC, Li CG, Wang HB, Luo HM, Li YP, Wang JF, et al. Immunogenicity of two different sequential schedules of inactivated polio vaccine followed by oral polio vaccine versus oral polio vaccine alone in healthy infants in China. Journal of the Pediatric Infectious Diseases Society 2016;5(3):287‐96. [DOI: 10.1093/jpids/piv017; PUBMED: 26407255] - DOI - PubMed
Linder 2000 {published data only}
-
- Linder N, Handsher R, German B, Sirota L, Bachman M, Zinger S, et al. Controlled trial of immune response of preterm infants to recombinant hepatitis B and inactivated poliovirus vaccines administered simultaneously shortly after birth. Archives of Disease in Childhood. Fetal and Neonatal Edition 2000;83(1):F24‐7. [DOI: 10.1136/fn.83.1.f24; PMC1721105; PUBMED: 10873167] - DOI - PMC - PubMed
Modlin 1997 {published data only}
-
- Modlin JF, Halsey NA, Thoms ML, Meschievitz CK, Patriarca PA. Humoral and mucosal immunity in infants induced by three sequential inactivated poliovirus vaccine‐live attenuated oral poliovirus vaccine immunization schedules. Baltimore Area Polio Vaccine Study Group. Journal of Infectious Diseases 1997;175 Suppl 1:S228‐34. [DOI: 10.1093/infdis/175.Supplement_1.S228; PUBMED: 9203721] - DOI - PubMed
O'Ryan 2015 {published data only}
-
- Brickley EB, Wieland‐Alter W, Connor RI, Ackerman ME, Boesch AW, Arita M, et al. Intestinal immunity to poliovirus following sequential trivalent inactivated polio vaccine/bivalent oral polio vaccine and trivalent inactivated polio vaccine‐only immunization schedules: analysis of an open‐label, randomized, controlled trial in Chilean infants. Clinical Infectious Diseases 2018;67(Suppl 1):S42‐50. [DOI: 10.1093/cid/ciy603; PMC6206105; PUBMED: 30376086] - DOI - PMC - PubMed
-
- O'Ryan M, Bandyopadhyay AS, Villena R, Espinoza M, Novoa J, Weldon WC, et al. Inactivated poliovirus vaccine given alone or in a sequential schedule with bivalent oral poliovirus vaccine in Chilean infants: a randomised, controlled, open‐label, phase 4, non‐inferiority study. Lancet. Infectious Diseases 2015;15(11):1273‐82. [DOI: 10.1016/S1473-3099(15)00219-4] - DOI - PubMed
Qiu 2017 {published data only}
-
- Qiu J, Yang Y, Huang L, Wang L, Jiang Z, Gong J, et al. Immunogenicity and safety evaluation of bivalent types 1 and 3 oral poliovirus vaccine by comparing different poliomyelitis vaccination schedules in China: a randomized controlled non‐inferiority clinical trial. Human Vaccines & Immunotherapeutics 2017;13(6):1359‐68. [DOI: 10.1080/21645515.2017.1288769; PMC5489289 ; PUBMED: 28362135] - DOI - PMC - PubMed
Ramsay 1994 {published data only}
Rennels 2000 {published data only}
-
- Laassri M, Lottenbach K, Belshe R, Rennels M, Plotkin S, Chumakov K. Analysis of reversions in the 5′‐untranslated region of attenuated poliovirus after sequential administration of inactivated and oral poliovirus vaccines. Journal of Infectious Diseases 2006;193(10):1344‐9. [DOI: 10.1086/503366; PUBMED: 16619180] - DOI - PubMed
-
- Rennels MB, Englund JA, Bernstein DI, Losonsky GA, Anderson EL, Pichichero ME, et al. Diminution of the anti‐polyribosylribitol phosphate response to a combined diphtheria‐tetanus‐acellular pertussis/Haemophilus influenzae type b vaccine by concurrent inactivated poliovirus vaccination. Pediatric Infectious Disease Journal 2000;19(5):417‐23. [DOI: 10.1097/00006454-200005000-00006; PUBMED: 10819337] - DOI - PubMed
Simasathien 1994 {published data only}
-
- Simasathien S, Migasena S, Beuvery C, Steenis G, Samakoses R, Pitisuttitham P, et al. Comparison of enhanced potency inactivated poliovirus vaccine (EIPV) versus standard oral poliovirus vaccine (OPV) in Thai infants. Scandinavian Journal of Infectious Diseases 1994;26(6):731‐8. [DOI: 10.3109/00365549409008643; PUBMED: 7747098] - DOI - PubMed
Sutter 1997 {published data only}
-
- Sutter RW, Suleiman AJ, Malankar PG, Mehta FR, Medany MA, Arif MA, et al. Sequential use of inactivated poliovirus vaccine followed by oral poliovirus vaccine in Oman. Journal of Infectious Diseases 1997;175 Suppl 1:S235‐40. [DOI: 10.1093/infdis/175.Supplement_1.S235; PUBMED: 9203722] - DOI - PubMed
Von Magnus 1984 {published data only}
West 2001 {published data only}
-
- West DJ, Rabalais GP, Watson B, Keyserling HL, Matthews H, Hesley TM. Antibody responses of healthy infants to concurrent administration of a bivalent Haemophilus influenzae type b‐hepatitis B vaccine with diphtheria‐tetanus‐pertussis, polio and measles‐mumps‐rubella vaccines. Biodrugs 2001;15(6):413‐8. [DOI: 10.2165/00063030-200115060-00007; PUBMED: 11520252] - DOI - PubMed
Yeh 2001 {published data only}
-
- Yeh SH, Ward JI, Partridge S, Marcy SM, Lee H, Jing J, et al. Safety and immunogenicity of a pentavalent diphtheria, tetanus, pertussis, hepatitis B and polio combination vaccine in infants. Pediatric Infectious Disease Journal 2001;20(10):973‐80. [DOI: 10.1097/00006454-200110000-00011; PUBMED: 11642632] - DOI - PubMed
References to studies excluded from this review
Li 2016b {published data only}
McCollough 1969 {published data only}
-
- McCollough RH, Glezen WP, Lamb GA, Chin TD. Booster effect of oral poliovaccine. Trials in persons previously immunized with inactivated vaccine. American Journal of Diseases of Children 1969;117(2):161‐8. [PUBMED: 4303394] - PubMed
Moǐseieva 2002 {published data only}
-
- Moǐseieva HV, Zadorozhna VI. Effectiveness of different strategies of vaccine prophylaxis for poliomyelitis in the Ukraine. Likars'ka sprava 2002;‐(2):85‐8. [PUBMED: 12073271] - PubMed
Swartz 1998 {published data only}
-
- Swartz TA, Handsher R, Manor Y, Stoeckel P, Barkay A, Mendelson E, et al. Immune response to an intercalated enhanced inactivated polio vaccine/oral polio vaccine programme in Israel: impact on the control of poliomyelitis. Vaccine 1998;16(20):2090‐5. [DOI: 10.1016/S0264-410X(98)00071-1; PUBMED: 9796069] - DOI - PubMed
Wattigney 2001 {published data only}
-
- Wattigney WA, Mootrey GT, Braun MM, Chen RT. Surveillance for poliovirus vaccine adverse events, 1991 to 1998: impact of a sequential vaccination schedule of inactivated poliovirus vaccine followed by oral poliovirus vaccine. Pediatrics 2001;107(5):E83. [DOI: 10.1542/peds.107.5.e83; PUBMED: 11331733] - DOI - PubMed
Ye 2018 {published data only}
-
- Ye H, Huang T, Ying ZF, Li GL, Che YC, Zhao ZM, et al. [Comparing the immunogenicity and safety of sequential inoculation of sIPV followed by bOPV (I + III) in different dosage forms] [株脊髓灰质炎灭活疫苗与不同剂型Ⅰ+Ⅲ型脊髓灰质炎减毒活疫苗序贯接种的免疫原性和安全性比较]. Zhonghua Yu Fang Yi Xue Za Zhi [Chinese Journal of Preventive Medicine] 2018;52(1):43‐9. [DOI: 10.3760/cma.j.issn.0253-9624.2018.01.009; PUBMED: 29334707] - DOI - PubMed
References to ongoing studies
NCT02412514 {published data only}
-
- NCT02412514. Intestinal and humoral immunity of sequential polio vaccination schedules. [Assessing the intestinal and humoral immunity of sequential schedules of inactivated poliovirus vaccine and bivalent oral poliovirus vaccine for routine childhood immunization in Bangladesh]. clinicaltrials.gov/ct2/show/NCT02412514 (first received 1 April 2015).
NCT03430349 {published data only}
-
- NCT03430349. Phase 1 novel live attenuated serotype 2 oral polio vaccine study in IPV primed adults (nOPV2M4a) [A phase 1 study to evaluate the safety and immunogenicity of 2 novel live attenuated serotype 2 oral poliovirus vaccines, in healthy adults previously primed with inactivated polio vaccine (IPV)]. clinicaltrials.gov/ct2/show/NCT03430349 (first received 21 January 2018).
NCT03614702 {published data only}
-
- NCT03614702. Clinic trial to evaluate the safety and immunogenicity by different sequential schedules of bOPV and IPV [Safety and immunogenicity evaluation of different sequential immunization schedules of type 1 + 2 bivalent oral poliovirus vaccine (bOPV) co‐administered with inactived poliovirus vaccine (IPV) in infants aged 2 months: a randomized, double blind, single center, parallel trial]. clinicaltrials.gov/show/nct03614702 (first received 3 March 2017).
Additional references
Aaby 2007
-
- Aaby P, Garly ML, Nielsen J, Ravn H, Martins C, Balé C, et al. Increased female‐male mortality ratio associated with inactivated polio and diphtheria‐tetanus‐pertussis vaccines: observations from vaccination trials in Guinea‐Bissau. Pediatric Infectious Disease Journal 2007;26(3):247‐52. [PUBMED: 17484223] - PubMed
Anand 2017
-
- Anand A, Molodecky NA, Pallansch MA, Sutter RW. Immunogenicity to poliovirus type 2 following two doses of fractional intradermal inactivated poliovirus vaccine: a novel dose sparing immunization schedule. Vaccine 2017;35(22):2993‐8. [DOI: 10.1016/j.vaccine.2017.03.008; PUBMED: 28434691] - DOI - PMC - PubMed
Asturias 2016
-
- Asturias EJ, Bandyopadhyay AS, Self S, Rivera L, Saez‐Llorens X, Lopez E, et al. Humoral and intestinal immunity induced by new schedules of bivalent oral poliovirus vaccine and one or two doses of inactivated poliovirus vaccine in Latin American infants: an open‐label randomised controlled trial. Lancet 2016;388(10040):158‐69. [DOI: 10.1016/S0140-6736(16)00703-0; PUBMED: 27212429] - DOI - PubMed
Bandyopadhyay 2018
-
- Bandyopadhyay AS, Modlin JF, Wenger J, Gast C. Immunogenicity of new primary immunization schedules with inactivated poliovirus vaccine and bivalent oral polio vaccine for the polio endgame: a review. Clinical Infectious Diseases 2018;67(Suppl 1):S35‐41. [DOI: 10.1093/cid/ciy633; PMC6206125 ; PUBMED: 30376081] - DOI - PMC - PubMed
Biffi 2003
-
- Biffi MR. Economic evaluation of the sequential schedule of polio vaccination in Italy [Valutazione economica dell’attivazione in Italia della schedula sequenziale per la vaccinazione antipoliomielitica]. PharmacoEconomics ‐ Italian Research Articles 2003;5(Suppl 1):47‐53. [DOI: 10.1007/BF03320615] - DOI
Brickley 2018
-
- Brickley EB, Wieland‐Alter W, Connor RI, Ackerman ME, Boesch AW, Arita M, et al. Intestinal immunity to poliovirus following sequential trivalent inactivated polio vaccine/bivalent oral polio vaccine and trivalent inactivated polio vaccine‐only immunization schedules: analysis of an open‐label, randomized, controlled trial in Chilean infants. Clinical Infectious Diseases 2018;67(Suppl 1):S42‐50. [DOI: 10.1093/cid/ciy603; NCT01841671; PMC6206105 ; PUBMED: 30376086] - DOI - PMC - PubMed
Campbell 2000
CDC 1997
-
- Centers for Disease Control and Prevention. Poliomyelitis prevention in the United States: introduction of a sequential vaccination schedule of inactivated poliovirus vaccine followed by oral poliovirus vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report. Recommendations and Reports 1997;46(RR‐3):1‐25. [www.cdc.gov/mmwr/PDF/rr/rr4603.pdf; PUBMED: 9026708] - PubMed
CDC 2015
-
- Centers for Disease Control and Prevention. Updates on CDC’s polio eradication efforts. www.cdc.gov/polio/updates/2015/2015‐1211.htm (accessed 9 December 2015).
Ciapponi 2011
-
- Ciapponi A, Glujovsky D, Bardach A, García Martí S, Comande D. EROS: a new software for early stage of systematic reviews. HTA for Health Systems Sustainability 8th Annual Meeting; 2011 Jun 27‐9; Rio de Janeiro (BR). 2011:66. [242; www.gapcongressos.com.br/eventos/z0082/documentos/Program_HTAi.pdf]
Ciapponi 2017
-
- Ciapponi A, Gibbons L. Global incidence of circulating vaccine‐derived poliovirus (cVDPV) during the period 2000‐2016: meta‐analysis [Incidencia mundial de poliovirus circulantes de origen vacunal (cVDPV) durante el período 2000‐2016: meta‐análisis]. XVI Reunión Anual de la Red Cochrane Iberoamericana: Síntesis y transferencia del conocimiento; 2017 Jun 5‐7; Medellín (CO). 2017.
Cockburn 1988
Cuba 2007
Cáceres 2001
de Oliveira 2000
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986; Vol. 7, issue 3:177‐88. [PUBMED: 3802833] - PubMed
Desai 2014
Deshpande 2003
-
- Deshpande JM, Nadkarni SS, Siddiqui ZA. Detection of MEF‐1 laboratory reference strain of poliovirus type 2 in children with poliomyelitis in India in 2002 & 2003. Indian Journal of Medical Research 2003;118:217‐23. [PUBMED: 14870793] - PubMed
Domingues 2014
-
- Domingues CM, Fátima Pereira S, Cunha Marreiros AC, Menezes N, Flannery B. Introduction of sequential inactivated polio vaccine‐oral polio vaccine schedule for routine infant immunization in Brazil's National Immunization Program. Journal of Infectious Diseases 2014;210 Suppl 1:S143‐51. [DOI: 10.1093/infdis/jit588; PMC4758462; PUBMED: 25316829] - DOI - PMC - PubMed
Dong 2016
-
- Dong SZ, Zhu WB. The role of Sabin inactivated poliovirus vaccine in the final phase of global polio eradication [萨宾灭活脊灰病毒疫苗在全球消灭脊髓灰质炎最后阶段的作用]. Zhonghua Yu Fang Yi Xue Za Zhi [Chinese Journal of Preventive Medicine] 2016;50(12):1032‐5. [DOI: 10.3760/cma.j.issn.0253-9624.2016.12.003; PUBMED: 28057104] - DOI - PubMed
Duintjer Tebbens 2006
Duintjer Tebbens 2015
EPOC 2011a
-
- Effective Practice, Organisation of Care. Cochrane Effective Practice and Organisation of Care Review Group: data collection checklist. bit.ly/1zXPg9y (accessed 12 May 2014).
EPOC 2011b
-
- Effective Practice, Organisation of Care. Draft EPOC methods paper: including Interrupted Time Series (ITS) designs in a EPOC review. bit.ly/1Eq6Zbf (accessed 16 March 2012).
EPOC 2011c
-
- Effective Practice, Organisation of Care. Presentation of data from EPOC studies (RCTs and CBAs). bit.ly/1y5dRDI (accessed 16 March 2012).
EPOC 2017
-
- Cochrane Effective Practice, Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/Resources... (accessed 7 April 2017).
Faden 1993
Fish 2016
Garon 2016
Gentile 2016
Glujovsky 2010
-
- Glujovsky D, Bardach A, García Martí S, Comande D, Ciapponi A. New software for early stage of systematic reviews. XVIII Cochrane Colloquium. The Joint Colloquium of the Cochrane and Campbell Collaborations; 2010 Oct 18‐22. 2010.
Glujovsky 2011
-
- Glujovsky D, Bardach A, García Martí S, Comande D, Ciapponi A. EROS: a new software for early stage of systematic reviews. Value in Health 2011;14(7):A564. [DOI: 10.1016/j.jval.2011.08.1689; PRM2] - DOI
Gold 1994
GPEI 2018
-
- Global Polio Eradication Initiative. Global wild poliovirus 2012 ‐ 2018. polioeradication.org/wp‐content/uploads/2018/01/global‐wild‐poliovirus‐2... (accessed 10 June 2018).
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 05 September 2015.
Grassly 2006
Grassly 2009
Grassly 2014
Guyatt 2011
Hawken 2012
Higgins 2003
Higgins 2011a
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JP, Altman DG, Sterne JAC, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
-
- Higgins JP, Deeks JJ, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hird 2012
Hultcrantz 2017
Jorba 2016
Kew 2004
Kohler 2002
Krugman 1977
-
- Krugman RD, Hardy GE Jr, Sellers C, Parkman PD, Witte JJ, Meyer BC, et al. Antibody persistence after primary immunization with trivalent oral poliovirus vaccine. Pediatrics 1977;60(1):80‐2. [PUBMED: 195265] - PubMed
Laassri 2005
Landaverde 2014
-
- Landaverde JM, Trumbo SP, Danovaro‐Holliday MC, Cochi SE, Gandhi R, Ruiz‐Matus C. Vaccine‐associated paralytic poliomyelitis in the postelimination era in Latin America and the Caribbean, 1992‐2011. Journal of Infectious Diseases 2014;209(9):1393‐402. [DOI: 10.1093/infdis/jit602; PUBMED: 24520126] - DOI - PubMed
Lewis 2017
-
- Lewis I, Ottosen A, Rubin J, Blanc DC, Zipursky S, Wootton E. A supply and demand management perspective on the accelerated global introductions of inactivated poliovirus vaccine in a constrained supply market. Journal of Infectious Diseases 2017;216(Suppl 1):S33‐9. [DOI: 10.1093/infdis/jiw550; PMC5853471; PUBMED: 28838159] - DOI - PMC - PubMed
Liberati 2009
-
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009; Vol. 6, issue 7:e1000100. [DOI: 10.1371/journal.pmed.1000100] - DOI - PMC - PubMed
Liu 2017
Lopalco 2017
Lund 2015
-
- Lund N, Andersen A, Hansen AS, Jepsen FS, Barbosa A, Biering‐Sørensen S, et al. The effect of oral polio vaccine at birth on infant mortality: a randomized trial. Clinical Infectious Diseases 2015;61(10):1504‐11. [DOI: 10.1093/cid/civ617; NCT00710983; PMC4614411; PUBMED: 26219694] - DOI - PMC - PubMed
Mascareñas 2005
McBean 1988
McCain 1979
-
- McCain LJ, McCleary R. The statistical analysis of the simple interrupted time‐series quasi‐experiment. In: Cook TD, Campbell DT editor(s). Quasi‐Experimentation: Design & Analysis Issues for Field Settings. Boston (MA): Houghton Mifflin, 1979:233‐93.
Miller 1996
-
- Miller MA, Sutter RW, Strebel PM, Hadler SC. Cost‐effectiveness of incorporating inactivated poliovirus vaccine into the routine childhood immunization schedule. JAMA 1996;276(12):967‐71. [PUBMED: 8805731] - PubMed
Minor 2009
Modlin 2019
Moher 2009
PAHO 2017
-
- Pan American Health Organization. Switch from tOPV to bOPV. www.paho.org/hq/index.php?option=com_content&view=article&id=11015:topv‐... (accessed 6 May 2017).
Patriarca 1991
-
- Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Reviews of Infectious Diseases 1991;13(5):926‐39. [PUBMED: 1660184] - PubMed
Peng 2018
Platt 2014
Resik 2013
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sartori 2015
Schünemann 2011b
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusion. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sterne 2011
-
- Sterne JA, Egger M, Moher D, on behalf of the Cochrane Bias Methods Group. Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Strebel 1995
-
- Strebel PM, Ion‐Nedelcu N, Baughman AL, Sutter RW, Cochi SL. Intramuscular injections within 30 days of immunization with oral poliovirus vaccine‐‐a risk factor for vaccine‐associated paralytic poliomyelitis. New England Journal of Medicine 1995;332(8):500‐6. [DOI: 10.1056/NEJM199502233320804; PUBMED: 7830731] - DOI - PubMed
Sutter 2000
-
- Sutter RW, Prevots DR, Cochi SL. Poliovirus vaccines. Progress toward global poliomyelitis eradication and changing routine immunization recommendations in the United States. Pediatric Clinics of North America 2000;47(2):287‐308. [PUBMED: 10761505] - PubMed
Sutter 2010
Tang 2018
-
- Tang G, Yin W, Cao Y, Tan L, Wu S, Cao Y, et al. Immunogenicity of sequential inactivated and oral poliovirus vaccines (OPV) versus inactivated poliovirus vaccine (IPV) alone in healthy infants: a systematic review and meta‐analysis. Human Vaccines & Immunotherapeutics 2018;14(11):2636‐43. [DOI: 10.1080/21645515.2018.1489188; PMC6314415 ; PUBMED: PMC6314415 ] - DOI - PMC - PubMed
Thompson 2015
Ukoumunne 1999
-
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area‐wide and organisation‐based interventions in health and health care: a systematic review. Health Technology Assessment 1999; Vol. 3, issue 5:iii‐92. [PUBMED: 10982317] - PubMed
WHO 2012
-
- WHO. Meeting of the Strategic Advisory Group of Experts on Immunization, November 2012 ‐ conclusions and recommendations. Relevé épidémiologique hebdomadaire 2013;88(1):1‐16. [www.who.int/wer/2013/wer8801.pdf; PUBMED: 23311010] - PubMed
WHO 2015a
-
- World Health Organization. Global Polio Eradication Initiative. Polio eradication & endgame strategic plan 2013–2018. polioeradication.org/wp‐content/uploads/2016/07/PEESP_EN_A4.pdf (accessed 17 January 2016).
WHO 2015b
-
- World Health Organization. Poliomyelitis. Key facts. www.who.int/en/news‐room/fact‐sheets/detail/poliomyelitis (accessed 17 January 2016).
WHO 2015c
-
- World Health Organization. Circulating vaccine‐derived poliovirus cases, 2000‐2015. https://drive.google.com/file/d/1sJqwL3jaZZcBjMWU3TXcMBBB7MKIKQ7H/view?u... (accessed 17 January 2016).
WHO 2015d
-
- World Health Organization. Global wild poliovirus 2013‐2018. polioeradication.org/wp‐content/uploads/2018/03/global‐wild‐poliovirus‐2... (accessed 6 March 2018).
WHO 2016
-
- World Health Organization. Polio vaccines: WHO position paper ‐ March, 2016. Relevé épidémiologique hebdomadaire 2016;91(12):145‐68. [www.who.int/wer/2016/wer9112.pdf?ua=1] - PubMed
WHO 2017
-
- World Health Organization. Polio case count. extranet.who.int/polis/public/CaseCount.aspx (accessed 6 May 2017).
WHO 2018
-
- World Health Organization. Global Polio Eradication Initiative. Global circulating vaccine‐derived poliovirus cases, 2000–17. polioeradication.org/polio‐today/polio‐now/this‐week/circulating‐vaccine... (accessed 6 June 2018).
WHO 2019
-
- World Health Organization. WHO vaccine‐preventable diseases: monitoring system. 2019 global summary. apps.who.int/immunization_monitoring/globalsummary/schedules (accessed 6 June 2018).
Wu 2018
Zhang 2016
Zhao 2017
-
- Zhao D, Ma R, Zhou T, Yang F, Wu J, Sun H, et al. Introduction of inactivated poliovirus vaccine and impact on vaccine‐associated paralytic poliomyelitis ‐ Beijing, China, 2014‐2016. Morbidity and Mortality Weekly Report 2017;66(49):1357‐61. [DOI: 10.15585/mmwr.mm6649a4; PMC5730215; PUBMED: 29240729] - DOI - PMC - PubMed
References to other published versions of this review
Ciapponi 2014
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

