Cellular senescence and senescence-associated secretory phenotype via the cGAS-STING signaling pathway in cancer
- PMID: 31799772
- PMCID: PMC7004529
- DOI: 10.1111/cas.14266
Cellular senescence and senescence-associated secretory phenotype via the cGAS-STING signaling pathway in cancer
Abstract
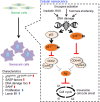
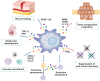
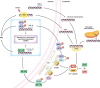
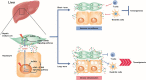
Cellular senescence is historically regarded as a tumor suppression mechanism to prevent damaged cells from aberrant proliferation in benign and premalignant tumors. However, recent findings have suggested that senescent cells contribute to tumorigenesis and age-associated pathologies through the senescence-associated secretory phenotype (SASP). Therefore, to control age-associated cancer, it is important to understand the molecular mechanisms of the SASP in the cancer microenvironment. New findings have suggested that the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) signaling pathway, a critical indicator of innate immune response, triggers the SASP in response to accumulation of cytoplasmic DNA (cytoplasmic chromatin fragments, mtDNA and cDNA) in senescent cells. Notably, the cGAS-STING signaling pathway promotes or inhibits tumorigenesis depending on the biological context in vivo, indicating that it may be a potential therapeutic target for cancer. Herein, we review the regulatory machinery and biological function of the SASP via the cGAS-STING signaling pathway in cancer.
Keywords: DNA damage; SASP; cGAS-STING; cellular senescence; tumorigenesis.
© 2019 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors have no conflict of interest.
Figures
Similar articles
-
Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence.Nat Cell Biol. 2017 Sep;19(9):1061-1070. doi: 10.1038/ncb3586. Epub 2017 Jul 31. Nat Cell Biol. 2017. PMID: 28759028 Free PMC article.
-
Autolysosomal degradation of cytosolic chromatin fragments antagonizes oxidative stress-induced senescence.J Biol Chem. 2020 Apr 3;295(14):4451-4463. doi: 10.1074/jbc.RA119.010734. Epub 2020 Feb 11. J Biol Chem. 2020. PMID: 32047109 Free PMC article.
-
cGAS-STING signaling in cancer immunity and immunotherapy.Biomed Pharmacother. 2021 Jan;133:110972. doi: 10.1016/j.biopha.2020.110972. Epub 2020 Nov 27. Biomed Pharmacother. 2021. PMID: 33254021 Review.
-
cGAS-STING mediates cytoplasmic mitochondrial-DNA-induced inflammatory signal transduction during accelerated senescence of pancreatic β-cells induced by metabolic stress.FASEB J. 2022 May;36(5):e22266. doi: 10.1096/fj.202101988R. FASEB J. 2022. PMID: 35357035
-
Innate immunosensing of DNA in cellular senescence.Curr Opin Immunol. 2019 Feb;56:31-36. doi: 10.1016/j.coi.2018.09.013. Epub 2018 Oct 5. Curr Opin Immunol. 2019. PMID: 30296662 Review.
Cited by
-
Extracellular vesicle-associated microRNA-30b-5p activates macrophages through the SIRT1/ NF-κB pathway in cell senescence.Front Immunol. 2022 Aug 31;13:955175. doi: 10.3389/fimmu.2022.955175. eCollection 2022. Front Immunol. 2022. PMID: 36119099 Free PMC article.
-
A novel prognostic model based on cellular senescence-related gene signature for bladder cancer.Front Oncol. 2022 Nov 23;12:937951. doi: 10.3389/fonc.2022.937951. eCollection 2022. Front Oncol. 2022. PMID: 36505846 Free PMC article.
-
Musashi-1 and miR-147 Precursor Interaction Mediates Synergistic Oncogenicity Induced by Co-Infection of Two Avian Retroviruses.Cells. 2022 Oct 21;11(20):3312. doi: 10.3390/cells11203312. Cells. 2022. PMID: 36291177 Free PMC article.
-
Expression of SASP, DNA Damage Response, and Cell Proliferation Factors in Early Gastric Neoplastic Lesions: Correlations and Clinical Significance.Pathol Oncol Res. 2022 Aug 19;28:1610401. doi: 10.3389/pore.2022.1610401. eCollection 2022. Pathol Oncol Res. 2022. PMID: 36061145 Free PMC article.
-
Role of the cGAS-STING Pathway in Aging-related Endothelial Dysfunction.Aging Dis. 2022 Dec 1;13(6):1901-1918. doi: 10.14336/AD.2022.0316. eCollection 2022 Dec 1. Aging Dis. 2022. PMID: 36465181 Free PMC article.
References
-
- Munoz‐Espin D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15:482‐496. - PubMed
-
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585‐621. - PubMed
-
- Serrano M, Lee H, Chin L, Cordon‐Cardo C, Beach D, DePinho RA. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27‐37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

