Zebrafish Vestigial Like Family Member 4b Is Required for Valvulogenesis Through Sequestration of Transcription Factor Myocyte Enhancer Factor 2c
- PMID: 31799250
- PMCID: PMC6874126
- DOI: 10.3389/fcell.2019.00277
Zebrafish Vestigial Like Family Member 4b Is Required for Valvulogenesis Through Sequestration of Transcription Factor Myocyte Enhancer Factor 2c
Abstract
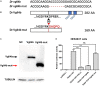
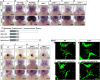
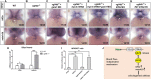
A variety of cardiac transcription factors/cofactors, signaling pathways, and downstream structural genes integrate to form the regulatory hierarchies to ensure proper cardiogenesis in vertebrate. Major interaction proteins of the transcription cofactor vestigial like family member 4 (VGLL4) include myocyte enhancer factor 2 (MEF2) and TEA domain transcription factors (TEAD), both of which play important roles in embryonic cardiac development and in adulthood. In this study, we identified that the deficiency of zebrafish vgll4b paralog, a unique family member expressed in developing heart, led to an impaired valve development. Mechanistically, in vgll4b mutant embryos the disruption of Vgll4b-Mef2c complex, rather than that of Vgll4b-Tead complex, resulted in an aberrant expression of krüppel-like factor 2a (klf2a) in endocardium. Such misexpression of klf2a eventually evoked the valvulogenesis defects. Our findings suggest that zebrafish Vgll4b plays an important role in modulating the transcription activity of Mef2c on klf2a during valve development in a blood-flow-independent manner.
Keywords: Mef2c; endocardium; klf2a; tead; valvulogenesis; vgll4b; zebrafish.
Copyright © 2019 Xue, Liu, Wen, Yang, Gao, Tao and Zhou.
Figures


Similar articles
-
The expression patterns of vestigial like family member 4 genes in zebrafish embryogenesis.Gene Expr Patterns. 2018 Jun;28:34-41. doi: 10.1016/j.gep.2018.02.001. Epub 2018 Feb 14. Gene Expr Patterns. 2018. PMID: 29454044
-
Oscillatory Flow Modulates Mechanosensitive klf2a Expression through trpv4 and trpp2 during Heart Valve Development.Curr Biol. 2015 May 18;25(10):1354-61. doi: 10.1016/j.cub.2015.03.038. Epub 2015 May 7. Curr Biol. 2015. PMID: 25959969
-
Reversing blood flows act through klf2a to ensure normal valvulogenesis in the developing heart.PLoS Biol. 2009 Nov;7(11):e1000246. doi: 10.1371/journal.pbio.1000246. Epub 2009 Nov 17. PLoS Biol. 2009. PMID: 19924233 Free PMC article.
-
Involvement of myocyte enhancer factor 2c in the pathogenesis of autism spectrum disorder.Heliyon. 2021 Apr 20;7(4):e06854. doi: 10.1016/j.heliyon.2021.e06854. eCollection 2021 Apr. Heliyon. 2021. PMID: 33981903 Free PMC article. Review.
-
Multiple Roles of Vestigial-Like Family Members in Tumor Development.Front Oncol. 2020 Jul 24;10:1266. doi: 10.3389/fonc.2020.01266. eCollection 2020. Front Oncol. 2020. PMID: 32793503 Free PMC article. Review.
Cited by
-
The Zebrafish Cardiac Endothelial Cell-Roles in Development and Regeneration.J Cardiovasc Dev Dis. 2021 May 1;8(5):49. doi: 10.3390/jcdd8050049. J Cardiovasc Dev Dis. 2021. PMID: 34062899 Free PMC article. Review.
-
Maternal vgll4a regulates zebrafish epiboly through Yap1 activity.Front Cell Dev Biol. 2024 Feb 20;12:1362695. doi: 10.3389/fcell.2024.1362695. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38444829 Free PMC article.
-
Multiple pkd and piezo gene family members are required for atrioventricular valve formation.Nat Commun. 2023 Jan 13;14(1):214. doi: 10.1038/s41467-023-35843-3. Nat Commun. 2023. PMID: 36639367 Free PMC article.
-
Significance of TEAD Family in Diagnosis, Prognosis and Immune Response for Ovarian Serous Carcinoma.Int J Gen Med. 2021 Oct 27;14:7133-7143. doi: 10.2147/IJGM.S336602. eCollection 2021. Int J Gen Med. 2021. PMID: 34737608 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

