BCG vaccination is associated with reduced malaria prevalence in children under the age of 5 years in sub-Saharan Africa
- PMID: 31798997
- PMCID: PMC6861070
- DOI: 10.1136/bmjgh-2019-001862
BCG vaccination is associated with reduced malaria prevalence in children under the age of 5 years in sub-Saharan Africa
Abstract
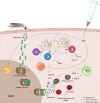
Introduction: Malaria continues to be a major cause of morbidity and mortality in sub-Saharan Africa (SSA) without effective interventions. Bacillus Calmette-Guérin (BCG) vaccine possesses protective non-specific effects, which extend beyond protection against tuberculosis. This study explores whether BCG is associated with protection against malaria in children under the age of 5 years in SSA.
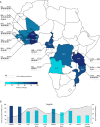
Methods: We used data from the Demographic Health Survey programme, including 34 206 children from 13 SSA countries. BCG status was taken from vaccination cards when present; if not, mother's recall was used. Presence of malaria was defined as a positive rapid diagnostic test. Maternally reported presence or absence of fever in the previous 2 weeks defined symptomatic status. Multilevel logistic regression was used to account for the two-stage cluster sampling method.
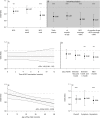
Results: Of the 34 206 children, 12 325 (36.0%) children were malaria positive and 29 766 (87.0%) were BCG vaccinated. After correction for relevant child, maternal and household factors, BCG vaccination was associated with a lower malaria prevalence (adjusted OR (aOR)=0.94, 95% CI 0.90 to 0.98), especially among children of whom BCG information was retrieved from a vaccination card (aORcard=0.88, 95% CI 0.82 to 0.94). Restricting the analysis to children from regions with suboptimal BCG coverage increased the association (aORcard=0.81, 95% CI 0.73 to 0.89). We observed an increasingly beneficial association with each month of age of the child (aORcard=0.996, 95% CI 0.993 to 0.999). BCG associations were similar for asymptomatic (aORcard=0.86, 95% CI 0.81 to 0.92) and symptomatic (aORcard=0.89, 95% CI 0.78 to 1.01) malaria.
Conclusions: BCG vaccination is associated with protection against malaria. This protection is highest in regions with suboptimal BCG coverage. These results indicate a possible role for timely BCG vaccination in the protection of malaria and its elimination by reducing the transmission reservoir. If confirmed in further research, our findings have substantial implications for global efforts to reduce malaria burden.
Keywords: DHS; bacillus Calmette-Guérin; heterologous effects; malaria.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form (www.icmje.org/coi_disclosure.pdf) and declare no support from companies for the submitted work; no financial relationships with companies that might have an interest in the submitted work in the previous 3 years; their spouses, partners or children have no financial relationships that may be relevant to the submitted work; no non-financial interests that may be relevant to the submitted work.
Figures
Comment in
-
Making sense of emerging evidence on the non-specific effects of the BCG vaccine on malaria risk and neonatal mortality.BMJ Glob Health. 2020 Mar 5;5(3):e002301. doi: 10.1136/bmjgh-2020-002301. eCollection 2020. BMJ Glob Health. 2020. PMID: 32201627 Free PMC article. No abstract available.
Similar articles
-
Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette-Guérin vaccination against tuberculosis.Health Technol Assess. 2013 Sep;17(37):1-372, v-vi. doi: 10.3310/hta17370. Health Technol Assess. 2013. PMID: 24021245 Free PMC article. Review.
-
Socio-demographic determinants of timely adherence to BCG, Penta3, measles, and complete vaccination schedule in Burkina Faso.Vaccine. 2013 Dec 17;32(1):96-102. doi: 10.1016/j.vaccine.2013.10.063. Epub 2013 Oct 30. Vaccine. 2013. PMID: 24183978
-
Drought and child vaccination coverage in 22 countries in sub-Saharan Africa: A retrospective analysis of national survey data from 2011 to 2019.PLoS Med. 2021 Sep 28;18(9):e1003678. doi: 10.1371/journal.pmed.1003678. eCollection 2021 Sep. PLoS Med. 2021. PMID: 34582463 Free PMC article.
-
Efficacy of BCG vaccination of the newborn: evaluation by a follow-up study of contacts in Bangui.Int J Epidemiol. 1995 Oct;24(5):1042-9. doi: 10.1093/ije/24.5.1042. Int J Epidemiol. 1995. PMID: 8557438
-
Bacillus Calmette-Guérin vaccination at birth: Effects on early childhood infections, growth, and development.Dan Med J. 2016 Nov;63(11):B5304. Dan Med J. 2016. PMID: 27808041 Review.
Cited by
-
Malaria and tuberculosis co-infection-a review.Oxf Open Immunol. 2023 Nov 15;4(1):iqad008. doi: 10.1093/oxfimm/iqad008. eCollection 2023. Oxf Open Immunol. 2023. PMID: 38089636 Free PMC article. Review.
-
Beneficial non-specific effects of live vaccines against COVID-19 and other unrelated infections.Lancet Infect Dis. 2023 Jan;23(1):e34-e42. doi: 10.1016/S1473-3099(22)00498-4. Epub 2022 Aug 26. Lancet Infect Dis. 2023. PMID: 36037824 Free PMC article. Review.
-
A cytokine super cyclone in COVID-19 patients with risk factors: the therapeutic potential of BCG immunization.Cytokine Growth Factor Rev. 2020 Aug;54:32-42. doi: 10.1016/j.cytogfr.2020.06.014. Epub 2020 Jul 1. Cytokine Growth Factor Rev. 2020. PMID: 32747157 Free PMC article. Review.
-
Hematopoietic stem and progenitor cells confer cross-protective trained immunity in mouse models.iScience. 2023 Aug 9;26(9):107596. doi: 10.1016/j.isci.2023.107596. eCollection 2023 Sep 15. iScience. 2023. PMID: 37664586 Free PMC article.
-
Malaria among under-five children in Ethiopia: a systematic review and meta-analysis.Malar J. 2022 Nov 16;21(1):338. doi: 10.1186/s12936-022-04370-9. Malar J. 2022. PMID: 36384533 Free PMC article.
References
-
- United Nations (UN) The millennium development goals report 2015. Available: http://www.un.org/millenniumgoals/2015_MDG_Report/pdf/MDG%202015%20rev%2... [Accessed 15 Dec 2017].
-
- World Health Organization (WHO) World malaria report 2018. Available: http://apps.who.int/iris/bitstream/handle/10665/275867/9789241565653-eng... [Accessed 15 Jan 2019].
LinkOut - more resources
Full Text Sources
Research Materials
