Endogenous Annexin-A1 Negatively Regulates Mast Cell-Mediated Allergic Reactions
- PMID: 31798445
- PMCID: PMC6865276
- DOI: 10.3389/fphar.2019.01313
Endogenous Annexin-A1 Negatively Regulates Mast Cell-Mediated Allergic Reactions
Abstract
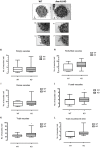
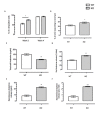
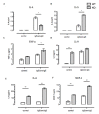
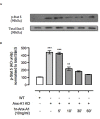
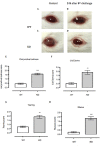
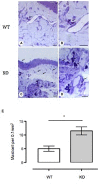
Mast cell stabilizers like cromoglycate and nedocromil are mainstream treatments for ocular allergy. Biochemical studies in vitro suggest that these drugs prevent mast cell degranulation through the release of Annexin-A1 (Anx-A1) protein. However, the direct effect of Anx-A1 gene deletion on mast cell function in vitro and in vivo is yet to be fully investigated. Hence, we aim to elucidate the role of Anx-A1 in mast cell function, both in vivo and in vitro, using a transgenic mouse model where the Anx-A1 gene has been deleted. Bone marrow-derived mast cells (BMDMCs) were cultured from wild-type animals and compared throughout their development to BMDMCs obtained from mice lacking the Anx-A1 gene. The mast cell differentiation, maturity, mediator, and cytokine release were explored using multiple biochemical techniques, such as Western blots, ELISA, and flow cytometry analysis. Electron microscopy was used to identify metachromatic granules content of cells. For in vivo studies, Balb/C wild-type and Anx-A1-deficient mice were divided into the following groups: group 1, a control receiving only saline, and group 2, which had been sensitized by prior exposure to short ragweed (SRW) pollen by topical contact with the conjunctival mucosae. Allergic conjunctivitis was evaluated blind after 24 h by trained observers scoring clinical signs. Electron micrographs of BMDMCs from Anx-A1-null mice revealed more vacuoles overall and more fused vacuoles than wild-type cells, suggesting enhanced secretory activity. Congruent with these observations, BMDMCs lacking the Anx-A1 gene released significantly increased amounts of histamine both spontaneously as well as in response to Ig-E-FcεRI cross-linking compared to those from wild-type mice. Interestingly, the spontaneous release of IL-5, IL-6, IL-9, and monocyte chemoattractant protein-1 (MCP-1) were also markedly increased with a greater production observed upon IgE cross-linking. This latter finding is congruent with augmented calcium mobilization in BMDMCs lacking the Anx-A1 gene. In vivo, when compared to wild-type animals, Anx-A1-deficient mice exposed to SRW pollen displayed exacerbated signs and symptoms of allergic conjunctivitis. Taken together, these results suggest Anx-A1 is an important non-redundant regulator of mast cell reactivity and particularly in allergen mediated allergic reactions.
Keywords: Annexin A1; allergic conjunctivitis model; bone marrow-derived mast cells; mast cell; transgenic mouse model.
Copyright © 2019 Sinniah, Yazid, Bena, Oliani, Perretti and Flower.
Figures
Similar articles
-
Anti-allergic cromones inhibit histamine and eicosanoid release from activated human and murine mast cells by releasing Annexin A1.PLoS One. 2013;8(3):e58963. doi: 10.1371/journal.pone.0058963. Epub 2013 Mar 18. PLoS One. 2013. PMID: 23527056 Free PMC article.
-
The role of the Annexin-A1/FPR2 system in the regulation of mast cell degranulation provoked by compound 48/80 and in the inhibitory action of nedocromil.Int Immunopharmacol. 2016 Mar;32:87-95. doi: 10.1016/j.intimp.2016.01.003. Epub 2016 Jan 21. Int Immunopharmacol. 2016. PMID: 26803520 Free PMC article.
-
Ocular anti-allergic compounds selectively inhibit human mast cell cytokines in vitro and conjunctival cell infiltration in vivo.Clin Exp Allergy. 2007 Nov;37(11):1648-56. doi: 10.1111/j.1365-2222.2007.02782.x. Epub 2007 Sep 17. Clin Exp Allergy. 2007. PMID: 17877767
-
A current appreciation of sites for pharmacological intervention in allergic conjunctivitis: effects of new topical ocular drugs.Acta Ophthalmol Scand Suppl. 1999;(228):33-7. doi: 10.1111/j.1600-0420.1999.tb01171.x. Acta Ophthalmol Scand Suppl. 1999. PMID: 10337430 Review.
-
Macrophage biology in the Anx-A1-/- mouse.Prostaglandins Leukot Essent Fatty Acids. 2005 Feb;72(2):95-103. doi: 10.1016/j.plefa.2004.10.008. Prostaglandins Leukot Essent Fatty Acids. 2005. PMID: 15626592 Review.
Cited by
-
Annexin A1 Mimetic Peptide Ac2-26 Modulates the Function of Murine Colonic and Human Mast Cells.Front Immunol. 2021 Sep 7;12:689484. doi: 10.3389/fimmu.2021.689484. eCollection 2021. Front Immunol. 2021. PMID: 34557187 Free PMC article.
-
Annexin Animal Models-From Fundamental Principles to Translational Research.Int J Mol Sci. 2021 Mar 26;22(7):3439. doi: 10.3390/ijms22073439. Int J Mol Sci. 2021. PMID: 33810523 Free PMC article. Review.
-
ANNEXIN A1: Roles in Placenta, Cell Survival, and Nucleus.Cells. 2022 Jun 29;11(13):2057. doi: 10.3390/cells11132057. Cells. 2022. PMID: 35805141 Free PMC article. Review.
-
Salivary Cystatin D Interactome in Patients with Systemic Mastocytosis: An Exploratory Study.Int J Mol Sci. 2023 Sep 27;24(19):14613. doi: 10.3390/ijms241914613. Int J Mol Sci. 2023. PMID: 37834061 Free PMC article.
-
The Multifaceted Role of Annexin A1 in Viral Infections.Cells. 2023 Apr 11;12(8):1131. doi: 10.3390/cells12081131. Cells. 2023. PMID: 37190040 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

