A review of the WHO malaria rapid diagnostic test product testing programme (2008-2018): performance, procurement and policy
- PMID: 31791354
- PMCID: PMC6889598
- DOI: 10.1186/s12936-019-3028-z
A review of the WHO malaria rapid diagnostic test product testing programme (2008-2018): performance, procurement and policy
Abstract
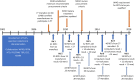
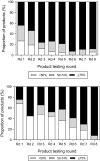
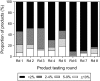
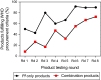
Malaria rapid diagnostic tests (RDTs) emerged in the early 1990s into largely unregulated markets, and uncertain field performance was a major concern for the acceptance of tests for malaria case management. This, combined with the need to guide procurement decisions of UN agencies and WHO Member States, led to the creation of an independent, internationally coordinated RDT evaluation programme aiming to provide comparative performance data of commercially available RDTs. Products were assessed against Plasmodium falciparum and Plasmodium vivax samples diluted to two densities, along with malaria-negative samples from healthy individuals, and from people with immunological abnormalities or non-malarial infections. Three measures were established as indicators of performance, (i) panel detection score (PDS) determined against low density panels prepared from P. falciparum and P. vivax wild-type samples, (ii) false positive rate, and (iii) invalid rate, and minimum criteria defined. Over eight rounds of the programme, 332 products were tested. Between Rounds 1 and 8, substantial improvements were seen in all performance measures. The number of products meeting all criteria increased from 26.8% (11/41) in Round 1, to 79.4% (27/34) in Round 8. While products submitted to further evaluation rounds under compulsory re-testing did not show improvement, those voluntarily resubmitted showed significant increases in P. falciparum (p = 0.002) and P. vivax PDS (p < 0.001), with more products meeting the criteria upon re-testing. Through this programme, the differentiation of products based on comparative performance, combined with policy changes has been influential in the acceptance of malaria RDTs as a case-management tool, enabling a policy of parasite-based diagnosis prior to treatment. Publication of product testing results has produced a transparent market allowing users and procurers to clearly identify appropriate products for their situation, and could form a model for introduction of other, broad-scale diagnostics.
Keywords: Malaria; Plasmodium falciparum; Plasmodium vivax; Product improvement; Rapid diagnostic tests.
Conflict of interest statement
JB and CK, declare no competing interests. SJ reports payment from WHO for writing the manuscript. MG reports agreements with WHO and FIND for performance of work to conduct statistical analysis. QC reports agreements with WHO and FIND for performance of work to characterize the parasite panel. SN, DM, WO and JL report payment from FIND for sample collection and characterization. JG reports payment of salary through a contract with FIND. JC reports payment of salary from WHO. IG and SI report grants with the Bill and Melinda Gates Foundation, UNITAID, UK Department for International Development, Australian Agency for International Development and USAID during the conduct of the study. PC and RRC report grants with WHO and FIND during the conduct of the study. DB reports past grants from The Bill and Melinda Gates Foundation and UNITAID during the conduct of the study.
Figures

Similar articles
-
Comparison of rapid diagnostic test Plasmotec Malaria-3, microscopy, and quantitative real-time PCR for diagnoses of Plasmodium falciparum and Plasmodium vivax infections in Mimika Regency, Papua, Indonesia.Malar J. 2015 Mar 5;14:103. doi: 10.1186/s12936-015-0615-5. Malar J. 2015. PMID: 25890368 Free PMC article.
-
Performance of three multi-species rapid diagnostic tests for diagnosis of Plasmodium falciparum and Plasmodium vivax malaria in Oromia Regional State, Ethiopia.Malar J. 2010 Oct 27;9:297. doi: 10.1186/1475-2875-9-297. Malar J. 2010. PMID: 20979601 Free PMC article.
-
Field and laboratory comparative evaluation of rapid malaria diagnostic tests versus traditional and molecular techniques in India.Malar J. 2010 Jul 5;9:191. doi: 10.1186/1475-2875-9-191. Malar J. 2010. PMID: 20602766 Free PMC article.
-
Malaria rapid diagnostic tests in endemic settings.Clin Microbiol Infect. 2013 May;19(5):399-407. doi: 10.1111/1469-0691.12151. Epub 2013 Feb 25. Clin Microbiol Infect. 2013. PMID: 23438048 Review.
-
Evaluating the efficacy of rapid diagnostic tests for imported malaria in high income countries: A systematic review.Int Emerg Nurs. 2022 Jan;60:101110. doi: 10.1016/j.ienj.2021.101110. Epub 2021 Dec 23. Int Emerg Nurs. 2022. PMID: 34953438 Review.
Cited by
-
Rethinking detection of pre-existing and intervening Plasmodium infections in malaria clinical trials.Front Immunol. 2022 Sep 20;13:1003452. doi: 10.3389/fimmu.2022.1003452. eCollection 2022. Front Immunol. 2022. PMID: 36203582 Free PMC article. Review.
-
Predicting Plasmodium falciparum infection status in blood using a multiplexed bead-based antigen detection assay and machine learning approaches.PLoS One. 2022 Sep 29;17(9):e0275096. doi: 10.1371/journal.pone.0275096. eCollection 2022. PLoS One. 2022. PMID: 36174056 Free PMC article.
-
Cost-effectiveness of sentinel screening of endemic diseases alongside malaria diagnosis: A case study in schistosomiasis.PLoS Negl Trop Dis. 2024 Jul 29;18(7):e0012339. doi: 10.1371/journal.pntd.0012339. eCollection 2024 Jul. PLoS Negl Trop Dis. 2024. PMID: 39074148 Free PMC article.
-
Things must not fall apart: the ripple effects of the COVID-19 pandemic on children in sub-Saharan Africa.Pediatr Res. 2021 Apr;89(5):1078-1086. doi: 10.1038/s41390-020-01174-y. Epub 2020 Sep 24. Pediatr Res. 2021. PMID: 32971527 Free PMC article. Review.
-
Diagnostic Methods for Non-Falciparum Malaria.Front Cell Infect Microbiol. 2021 Jun 17;11:681063. doi: 10.3389/fcimb.2021.681063. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34222049 Free PMC article. Review.
References
-
- WHO . World malaria report. Geneva: World Health Organization; 2018.
-
- WHO. New perspectives: malaria diagnosis. Report of joint WHO/USAID informal consultation 25–27 October. Geneva: World Health Organization; 2000.
-
- Thepsamarn P, Prayoollawongsa N, Puksupa P, Puttoom P, Thaidumrong P, Wongchai S, et al. The ICT malaria Pf: a simple, rapid dipstick test for the diagnosis of Plasmodium falciparum malaria at the Thai-Myanmar border. Southeast Asian J Trop Med Public Health. 1997;28:723–726. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

