Radiation-Induced Normal Tissue Damage: Oxidative Stress and Epigenetic Mechanisms
- PMID: 31781332
- PMCID: PMC6875293
- DOI: 10.1155/2019/3010342
Radiation-Induced Normal Tissue Damage: Oxidative Stress and Epigenetic Mechanisms
Abstract
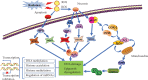
Radiotherapy (RT) is currently one of the leading treatments for various cancers; however, it may cause damage to healthy tissue, with both short-term and long-term side effects. Severe radiation-induced normal tissue damage (RINTD) frequently has a significant influence on the progress of RT and the survival and prognosis of patients. The redox system has been shown to play an important role in the early and late effects of RINTD. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are the main sources of RINTD. The free radicals produced by irradiation can upregulate several enzymes including nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase), lipoxygenases (LOXs), nitric oxide synthase (NOS), and cyclooxygenases (COXs). These enzymes are expressed in distinct ways in various cells, tissues, and organs and participate in the RINTD process through different regulatory mechanisms. In recent years, several studies have demonstrated that epigenetic modulators play an important role in the RINTD process. Epigenetic modifications primarily contain noncoding RNA regulation, histone modifications, and DNA methylation. In this article, we will review the role of oxidative stress and epigenetic mechanisms in radiation damage, and explore possible prophylactic and therapeutic strategies for RINTD.
Copyright © 2019 Jinlong Wei et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Reduction-oxidation (redox) system in radiation-induced normal tissue injury: molecular mechanisms and implications in radiation therapeutics.Clin Transl Oncol. 2018 Aug;20(8):975-988. doi: 10.1007/s12094-017-1828-6. Epub 2018 Jan 9. Clin Transl Oncol. 2018. PMID: 29318449 Review.
-
Targeting of cellular redox metabolism for mitigation of radiation injury.Life Sci. 2020 Jun 1;250:117570. doi: 10.1016/j.lfs.2020.117570. Epub 2020 Mar 20. Life Sci. 2020. PMID: 32205088 Review.
-
Ionizing radiation-induced oxidative stress, epigenetic changes and genomic instability: the pivotal role of mitochondria.Int J Radiat Biol. 2015 Jan;91(1):1-12. doi: 10.3109/09553002.2014.934929. Int J Radiat Biol. 2015. PMID: 24937368 Review.
-
Low-Dose Ionizing Radiation Exposure, Oxidative Stress and Epigenetic Programing of Health and Disease.Radiat Res. 2017 Oct;188(4.2):525-538. doi: 10.1667/RR14587.1. Epub 2017 Jul 28. Radiat Res. 2017. PMID: 28753061 Review.
-
NADPH Oxidase as a Target for Modulation of Radiation Response; Implications to Carcinogenesis and Radiotherapy.Curr Mol Pharmacol. 2019;12(1):50-60. doi: 10.2174/1874467211666181010154709. Curr Mol Pharmacol. 2019. PMID: 30318012 Review.
Cited by
-
CLIC4 regulates radioresistance of nasopharyngeal carcinoma by iNOS after γ-rays but not carbon ions irradiation.Am J Cancer Res. 2020 May 1;10(5):1400-1415. eCollection 2020. Am J Cancer Res. 2020. PMID: 32509387 Free PMC article.
-
Mesenchymal Stem Cells for Mitigating Radiotherapy Side Effects.Cells. 2021 Feb 1;10(2):294. doi: 10.3390/cells10020294. Cells. 2021. PMID: 33535574 Free PMC article. Review.
-
Radiation Induced Skin Fibrosis (RISF): Opportunity for Angiotensin II-Dependent Intervention.Int J Mol Sci. 2024 Jul 29;25(15):8261. doi: 10.3390/ijms25158261. Int J Mol Sci. 2024. PMID: 39125831 Free PMC article. Review.
-
Cooperation effects of radiation and ferroptosis on tumor suppression and radiation injury.Front Cell Dev Biol. 2022 Sep 13;10:951116. doi: 10.3389/fcell.2022.951116. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36176274 Free PMC article. Review.
-
Revolutionizing Radiotoxicity Management with Mesenchymal Stem Cells and Their Derivatives: A Focus on Radiation-Induced Cystitis.Int J Mol Sci. 2023 May 22;24(10):9068. doi: 10.3390/ijms24109068. Int J Mol Sci. 2023. PMID: 37240415 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical