The myofibroblast, biological activities and roles in eye repair and fibrosis. A focus on healing mechanisms in avascular cornea
- PMID: 31767967
- PMCID: PMC7002667
- DOI: 10.1038/s41433-019-0684-8
The myofibroblast, biological activities and roles in eye repair and fibrosis. A focus on healing mechanisms in avascular cornea
Abstract
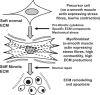
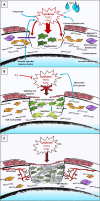
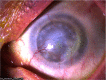
Tissue healing is one of the mysteries of modern medicine. Healing involves complex processes and many cellular types, amongst which the myofibroblast plays a major role. In the eye, when needed, myofibroblasts can be found from the cornea to the retina, derived from a wide variety of different cells, and aimed at effectively repairing tissue damage. Myofibroblast differentiation requires transforming growth factor (TGF)-β1, the presence of specific extracellular matrix components such as the ED-A domain of fibronectin, and mechanical tension. Control of this process may, in some cases, be abnormal leading to development of fibrotic tissue, which alters and compromises the integrity of the original tissue. The eye is no exception to this rule with normal visual function, a highly demanding process, only possible in a fully integrated organ. The cornea, a transparent protective tissue and first dioptre of the eye, has the particularity of being entirely avascular and very richly innervated under normal physiological conditions. However, these anatomical features do not prevent it from developing myofibroblasts in the event of a deep corneal lesion. Activated by growth factors such as TGF-β1 and platelet-derived growth factor from the aqueous humour, tears or corneal epithelial cells, myofibroblasts can cause corneal scarring, sometimes with devastating consequences. Understanding the factors involved in healing and its signalling pathways, will potentially enable us to control corneal healing in the future, and thus avoid fibrotic ocular surface disease and the blindness that this may induce. Currently, this issue is the subject of very active research and development with the aim of discovering new antifibrotic therapies.
摘要: 组织修复/愈合是现代医学的奥秘之一。修复/愈合涉及复杂的过程和多种细胞类型, 其中肌成纤维细胞起主要作用。必要时, 在眼内从角膜到视网膜都能发现源自不同种类细胞的肌成纤维细胞, 作用在于有效地修复组织损伤。肌成纤维细胞的分化需要转化生长因子 (TGF) -β1和特定的细胞外基质成分, 如纤维连接蛋白的ED-A结构域和机械张力。在某些情况下, 对这个过程的干预可能是不正常的, 导致纤维组织异常增殖, 这将会改变和破坏原始组织的完整性。眼睛也不例外, 正常的视觉功能, 是一个要求极高的过程, 只有在一个结构完整的器官才能执行。角膜是一层透明的保护组织, 也是眼的第一层屈光组织, 在正常生理条件下具有完全无血管和受丰富神经支配的特性。然而这些解剖特点并不能阻止它在深部角膜损伤转变为肌成纤维细胞。肌成纤维细胞被生长因子如TGF-β1和来自房水、泪液或角膜上皮细胞的血小板衍生生长因子—可导致角膜瘢痕形成, 有时会带来毁灭性的后果。了解角膜愈合的相关因素及其信号通路传导途径, 将有助于我们在未来控制角膜的愈合过程, 从而避免纤维性眼表疾病和其可能的致盲。这个问题是当前研究中的热门课题, 其目的是发现新的抗纤维疗法。.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
The Corneal Basement Membranes and Stromal Fibrosis.Invest Ophthalmol Vis Sci. 2018 Aug 1;59(10):4044-4053. doi: 10.1167/iovs.18-24428. Invest Ophthalmol Vis Sci. 2018. PMID: 30098200 Free PMC article. Review.
-
Corneal epithelial basement membrane: Structure, function and regeneration.Exp Eye Res. 2020 May;194:108002. doi: 10.1016/j.exer.2020.108002. Epub 2020 Mar 13. Exp Eye Res. 2020. PMID: 32179076 Free PMC article. Review.
-
The corneal fibrosis response to epithelial-stromal injury.Exp Eye Res. 2016 Jan;142:110-8. doi: 10.1016/j.exer.2014.09.012. Exp Eye Res. 2016. PMID: 26675407 Free PMC article. Review.
-
Corneal wound healing.Exp Eye Res. 2020 Aug;197:108089. doi: 10.1016/j.exer.2020.108089. Epub 2020 Jun 15. Exp Eye Res. 2020. PMID: 32553485 Free PMC article. Review.
-
Modulation of Smad signaling by non-TGFβ components in myofibroblast generation during wound healing in corneal stroma.Exp Eye Res. 2016 Jan;142:40-8. doi: 10.1016/j.exer.2014.12.015. Exp Eye Res. 2016. PMID: 26675402 Review.
Cited by
-
Macrophages modulate fibrosis during newt lens regeneration.Stem Cell Res Ther. 2024 May 14;15(1):141. doi: 10.1186/s13287-024-03740-1. Stem Cell Res Ther. 2024. PMID: 38745238 Free PMC article.
-
Corneal injury repair and the potential involvement of ZEB1.Eye Vis (Lond). 2024 Jun 1;11(1):20. doi: 10.1186/s40662-024-00387-0. Eye Vis (Lond). 2024. PMID: 38822380 Free PMC article. Review.
-
BMP3 inhibits TGFβ2-mediated myofibroblast differentiation during wound healing of the embryonic cornea.NPJ Regen Med. 2022 Jul 25;7(1):36. doi: 10.1038/s41536-022-00232-9. NPJ Regen Med. 2022. PMID: 35879352 Free PMC article.
-
Macrophages modulate fibrosis during newt lens regeneration.Res Sq [Preprint]. 2023 Nov 25:rs.3.rs-3603645. doi: 10.21203/rs.3.rs-3603645/v1. Res Sq. 2023. Update in: Stem Cell Res Ther. 2024 May 14;15(1):141. doi: 10.1186/s13287-024-03740-1. PMID: 38045376 Free PMC article. Updated. Preprint.
-
Gene Expression Profile of Vascular Endothelial Growth Factors (VEGFs) and Platelet-derived Growth Factors (PDGFs) in the Normal Cornea.In Vivo. 2021 Mar-Apr;35(2):805-813. doi: 10.21873/invivo.12321. In Vivo. 2021. PMID: 33622873 Free PMC article.
References
-
- Yazdani S, Bansal R, Prakash J. Drug targeting to myofibroblasts: implications for fibrosis and cancer. Adv Drug Deliv Rev. 2017;121:101–16. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

