Bi- and tri-valent T cell engagers deplete tumour-associated macrophages in cancer patient samples
- PMID: 31753017
- PMCID: PMC6873687
- DOI: 10.1186/s40425-019-0807-6
Bi- and tri-valent T cell engagers deplete tumour-associated macrophages in cancer patient samples
Abstract
Background: Tumour-associated macrophages (TAMs) are often implicated in cancer progression but can also exert anti-tumour activities. Selective eradication of cancer-promoting (M2-like) TAM subsets is a highly sought-after goal. Here, we have devised a novel strategy to achieve selective TAM depletion, involving the use of T cell engagers to direct endogenous T cell cytotoxicity towards specific M2-like TAMs. To avoid "on-target off-tumour" toxicities, we have explored localising expression of the T cell engagers to the tumour with enadenotucirev (EnAd), an oncolytic adenovirus in Phase I/II clinical trials.
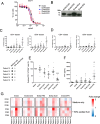
Method: A panel of bi- and tri-valent T cell engagers (BiTEs/TriTEs) was constructed, recognising CD3ε on T cells and CD206 or folate receptor β (FRβ) on M2-like macrophages. Initial characterisation of BiTE/TriTE activity and specificity was performed with M1- and M2-polarised monocyte-derived macrophages and autologous lymphocytes from healthy human peripheral blood donors. T cell engagers were inserted into the genome of EnAd, and oncolytic activity and BiTE secretion assessed with DLD-1 tumour cells. Clinically-relevant ex vivo models (whole malignant ascites from cancer patients) were employed to assess the efficacies of the free- and virally-encoded T cell engagers.
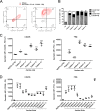
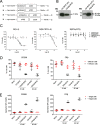
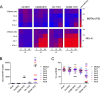
Results: T cells activated by the CD206- and FRβ-targeting BiTEs/TriTEs preferentially killed M2- over M1-polarised autologous macrophages, with EC50 values in the nanomolar range. A TriTE with bivalent CD3ε binding - the first of its kind - demonstrated enhanced potency whilst retaining target cell selectivity, whereas a CD28-containing TriTE elicited non-specific T cell activation. In immunosuppressive malignant ascites, both free and EnAd-encoded T cell engagers triggered endogenous T cell activation and IFN-γ production, leading to increased T cell numbers and depletion of CD11b+CD64+ ascites macrophages. Strikingly, surviving macrophages exhibited a general increase in M1 marker expression, suggesting microenvironmental repolarisation towards a pro-inflammatory state.
Conclusions: This study is the first to achieve selective depletion of specific M2-like macrophage subsets, opening the possibility of eradicating cancer-supporting TAMs whilst sparing those with anti-tumour potential. Targeted TAM depletion with T cell engager-armed EnAd offers a powerful therapeutic approach combining direct cancer cell cytotoxicity with reversal of immune suppression.
Keywords: Bispecific T cell engagers; Oncolytic virus; Tumour microenvironment; Tumour-associated macrophages.
Conflict of interest statement
LWS, BRC and KDF own equity or share options in PsiOxus Therapeutics (Oxford, UK), which is leading clinical development of EnAd and its derivatives.
Figures



Similar articles
-
Oncolytic herpesvirus expressing PD-L1 BiTE for cancer therapy: exploiting tumor immune suppression as an opportunity for targeted immunotherapy.J Immunother Cancer. 2021 Mar;9(4):e001292. doi: 10.1136/jitc-2020-001292. J Immunother Cancer. 2021. PMID: 33820820 Free PMC article.
-
Tumor cell-released autophagosomes (TRAPs) promote immunosuppression through induction of M2-like macrophages with increased expression of PD-L1.J Immunother Cancer. 2018 Dec 18;6(1):151. doi: 10.1186/s40425-018-0452-5. J Immunother Cancer. 2018. PMID: 30563569 Free PMC article.
-
Revolutionizing cancer treatment: the power of bi- and tri-specific T-cell engagers in oncolytic virotherapy.Front Immunol. 2024 Feb 22;15:1343378. doi: 10.3389/fimmu.2024.1343378. eCollection 2024. Front Immunol. 2024. PMID: 38464532 Free PMC article. Review.
-
Oncolytic adenovirus expressing bispecific antibody targets T-cell cytotoxicity in cancer biopsies.EMBO Mol Med. 2017 Aug;9(8):1067-1087. doi: 10.15252/emmm.201707567. EMBO Mol Med. 2017. PMID: 28634161 Free PMC article.
-
The Multifaceted Role of Macrophages in Oncolytic Virotherapy.Viruses. 2021 Aug 9;13(8):1570. doi: 10.3390/v13081570. Viruses. 2021. PMID: 34452439 Free PMC article. Review.
Cited by
-
Immunovirotherapy: The role of antibody based therapeutics combination with oncolytic viruses.Front Immunol. 2022 Oct 13;13:1012806. doi: 10.3389/fimmu.2022.1012806. eCollection 2022. Front Immunol. 2022. PMID: 36311790 Free PMC article. Review.
-
Tackling HLA Deficiencies Head on with Oncolytic Viruses.Cancers (Basel). 2021 Feb 10;13(4):719. doi: 10.3390/cancers13040719. Cancers (Basel). 2021. PMID: 33578735 Free PMC article. Review.
-
Germline mutations and blood malignancy (Review).Oncol Rep. 2021 Jan;45(1):49-57. doi: 10.3892/or.2020.7846. Epub 2020 Nov 11. Oncol Rep. 2021. PMID: 33200226 Review.
-
The landscape of bispecific T cell engager in cancer treatment.Biomark Res. 2021 May 26;9(1):38. doi: 10.1186/s40364-021-00294-9. Biomark Res. 2021. PMID: 34039409 Free PMC article. Review.
-
Oncolytic adenovirus: A tool for reversing the tumor microenvironment and promoting cancer treatment (Review).Oncol Rep. 2021 Apr;45(4):49. doi: 10.3892/or.2021.8000. Epub 2021 Mar 24. Oncol Rep. 2021. PMID: 33760203 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
