Peritumoral administration of DRibbles-pulsed antigen-presenting cells enhances the antitumor efficacy of anti-GITR and anti-PD-1 antibodies via an antigen presenting independent mechanism
- PMID: 31747946
- PMCID: PMC6865022
- DOI: 10.1186/s40425-019-0786-7
Peritumoral administration of DRibbles-pulsed antigen-presenting cells enhances the antitumor efficacy of anti-GITR and anti-PD-1 antibodies via an antigen presenting independent mechanism
Abstract
Background: TNF receptor family agonists and checkpoint blockade combination therapies lead to minimal tumor clearance of poorly immunogenic tumors. Therefore, a need to enhance the efficacy of this combination therapy arises. Antigen-presenting cells (APCs) present antigen to T cells and steer the immune response through chemokine and cytokine secretion. DRibbles (DR) are tumor-derived autophagosomes containing tumor antigens and innate inflammatory adjuvants.
Methods: Using preclinical murine lung and pancreatic cancer models, we assessed the triple combination therapy of GITR agonist and PD-1 blocking antibodies with peritumoral injections of DRibbles-pulsed-bone marrow cells (BMCs), which consisted mainly of APCs, or CD103+ cross-presenting dendritic cells (DCs). Immune responses were assessed by flow cytometry. FTY720 was used to prevent T-cell egress from lymph nodes to assess lymph node involvement, and MHC-mismatched-BMCs were used to assess the necessity of antigen presentation by the peritumorally-injected DR-APCs.
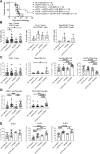
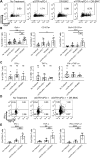
Results: Tritherapy increased survival and cures in tumor-bearing mice compared to combined antibody therapy or peritumoral DR-BMCs alone. Peritumorally-injected BMCs remained within the tumor for at least 14 days and tritherapy efficacy was dependent on both CD4+ and CD8+ T cells. Although the overall percent of tumor-infiltrating T cells remained similar, tritherapy increased the ratio of effector CD4+ T cells-to-regulatory T cells, CD4+ T-cell cytokine production and proliferation, and CD8+ T-cell cytolytic activity in the tumor. Despite tritherapy-induced T-cell activation and cytolytic activity in lymph nodes, this T-cell activation was not required for tumor regression and enhanced survival. Replacement of DR-BMCs with DR-pulsed-DCs in the tritherapy led to similar antitumor effects, whereas replacement with DRibbles was less effective but delayed tumor growth. Interestingly, peritumoral administration of DR-pulsed MHC-mismatched-APCs in the tritherapy led to similar antitumor effects as MHC-matched-APCs, indicating that the observed enhanced antitumor effect was mediated independently of antigen presentation by the administered APCs.
Conclusions: Overall, these results demonstrate that peritumoral DR-pulsed-BMC/DC administration synergizes with GITR agonist and PD-1 blockade to locally modulate and sustain tumor effector T-cell responses independently of T cell priming and perhaps through innate inflammatory modulations mediated by the DRibbles adjuvant. We offer a unique approach to modify the tumor microenvironment to benefit T-cell-targeted immunotherapies.
Keywords: Antigen presenting cells; Dendritic cells; GITR; PD-1; Peritumoral injection; Tumor microenvironment.
Conflict of interest statement
HMH is a cofounder of UbiVac, a biotech company that has licensed the DRibbles intellectual property. The other authors declare that they have no competing interests.
Figures





Similar articles
-
Combined PD-1 blockade and GITR triggering induce a potent antitumor immunity in murine cancer models and synergizes with chemotherapeutic drugs.J Transl Med. 2014 Feb 7;12:36. doi: 10.1186/1479-5876-12-36. J Transl Med. 2014. PMID: 24502656 Free PMC article.
-
Agonist anti-GITR antibody significantly enhances the therapeutic efficacy of Listeria monocytogenes-based immunotherapy.J Immunother Cancer. 2017 Aug 15;5(1):64. doi: 10.1186/s40425-017-0266-x. J Immunother Cancer. 2017. PMID: 28807056 Free PMC article.
-
GITR ligand fusion protein agonist enhances the tumor antigen-specific CD8 T-cell response and leads to long-lasting memory.J Immunother Cancer. 2017 Jun 20;5:47. doi: 10.1186/s40425-017-0247-0. eCollection 2017. J Immunother Cancer. 2017. PMID: 28649380 Free PMC article.
-
Clinically feasible approaches to potentiating cancer cell-based immunotherapies.Hum Vaccin Immunother. 2015;11(4):851-69. doi: 10.1080/21645515.2015.1009814. Hum Vaccin Immunother. 2015. PMID: 25933181 Free PMC article. Review.
-
The ABCs of Antigen Presentation by Stromal Non-Professional Antigen-Presenting Cells.Int J Mol Sci. 2021 Dec 23;23(1):137. doi: 10.3390/ijms23010137. Int J Mol Sci. 2021. PMID: 35008560 Free PMC article. Review.
Cited by
-
ITGB2 as a prognostic indicator and a predictive marker for immunotherapy in gliomas.Cancer Immunol Immunother. 2022 Mar;71(3):645-660. doi: 10.1007/s00262-021-03022-2. Epub 2021 Jul 27. Cancer Immunol Immunother. 2022. PMID: 34313821 Free PMC article.
-
Perspectives in Melanoma: meeting report from the Melanoma Bridge (December 2nd - 4th, 2021, Italy).J Transl Med. 2022 Sep 4;20(1):391. doi: 10.1186/s12967-022-03592-4. J Transl Med. 2022. PMID: 36058945 Free PMC article.
-
Biomaterials Facilitating Dendritic Cell-Mediated Cancer Immunotherapy.Adv Sci (Weinh). 2023 Jun;10(18):e2301339. doi: 10.1002/advs.202301339. Epub 2023 Apr 23. Adv Sci (Weinh). 2023. PMID: 37088780 Free PMC article. Review.
-
Regulation of autophagy fires up the cold tumor microenvironment to improve cancer immunotherapy.Front Immunol. 2022 Oct 10;13:1018903. doi: 10.3389/fimmu.2022.1018903. eCollection 2022. Front Immunol. 2022. PMID: 36300110 Free PMC article. Review.
-
Unveiling the potential of HSPA4: a comprehensive pan-cancer analysis of HSPA4 in diagnosis, prognosis, and immunotherapy.Aging (Albany NY). 2024 Jan 31;16(3):2517-2541. doi: 10.18632/aging.205496. Epub 2024 Jan 31. Aging (Albany NY). 2024. PMID: 38305786 Free PMC article.
References
-
- Linch SN, Kasiewicz MJ, McNamara MJ, Hilgart-Martiszus IF, Farhad M, Redmond WL. Combination OX40 agonism/CTLA-4 blockade with HER2 vaccination reverses T-cell anergy and promotes survival in tumor-bearing mice. Proc Natl Acad Sci U S A. 2016;113(3):E319–E327. doi: 10.1073/pnas.1510518113. - DOI - PMC - PubMed
-
- Ramirez-Montagut T, Chow A, Hirschhorn-Cymerman D, Terwey TH, Kochman AA, Lu S, et al. Glucocorticoid-induced TNF receptor family related gene activation overcomes tolerance/ignorance to melanoma differentiation antigens and enhances antitumor immunity. J Immunol. 2006;176(11):6434–6442. doi: 10.4049/jimmunol.176.11.6434. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
