Structure of an endosomal signaling GPCR-G protein-β-arrestin megacomplex
- PMID: 31740855
- PMCID: PMC7108872
- DOI: 10.1038/s41594-019-0330-y
Structure of an endosomal signaling GPCR-G protein-β-arrestin megacomplex
Abstract
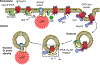
Classically, G-protein-coupled receptors (GPCRs) are thought to activate G protein from the plasma membrane and are subsequently desensitized by β-arrestin (β-arr). However, some GPCRs continue to signal through G protein from internalized compartments, mediated by a GPCR-G protein-β-arr 'megaplex'. Nevertheless, the molecular architecture of the megaplex remains unknown. Here, we present its cryo-electron microscopy structure, which shows simultaneous engagement of human G protein and bovine β-arr to the core and phosphorylated tail, respectively, of a single active human chimeric β2-adrenergic receptor with the C-terminal tail of the arginine vasopressin type 2 receptor (β2V2R). All three components adopt their canonical active conformations, suggesting that a single megaplex GPCR is capable of simultaneously activating G protein and β-arr. Our findings provide a structural basis for GPCR-mediated sustained internalized G protein signaling.
Conflict of interest statement
Competing Interests Statement
The authors declare no competing interests.
Figures


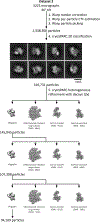

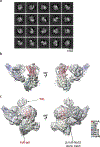



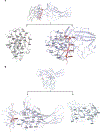
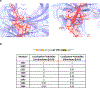
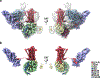
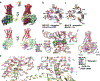


Similar articles
-
Signaling at the endosome: cryo-EM structure of a GPCR-G protein-beta-arrestin megacomplex.FEBS J. 2021 Apr;288(8):2562-2569. doi: 10.1111/febs.15773. Epub 2021 Mar 8. FEBS J. 2021. PMID: 33605032 Free PMC article.
-
GPCR-G Protein-β-Arrestin Super-Complex Mediates Sustained G Protein Signaling.Cell. 2016 Aug 11;166(4):907-919. doi: 10.1016/j.cell.2016.07.004. Epub 2016 Aug 4. Cell. 2016. PMID: 27499021 Free PMC article.
-
β-Arrestin-dependent and -independent endosomal G protein activation by the vasopressin type 2 receptor.Elife. 2023 Oct 19;12:RP87754. doi: 10.7554/eLife.87754. Elife. 2023. PMID: 37855711 Free PMC article.
-
Structural features of activated GPCR signaling complexes.Curr Opin Struct Biol. 2020 Aug;63:82-89. doi: 10.1016/j.sbi.2020.04.008. Epub 2020 May 30. Curr Opin Struct Biol. 2020. PMID: 32485565 Review.
-
Structural insights into emergent signaling modes of G protein-coupled receptors.J Biol Chem. 2020 Aug 14;295(33):11626-11642. doi: 10.1074/jbc.REV120.009348. Epub 2020 Jun 22. J Biol Chem. 2020. PMID: 32571882 Free PMC article. Review.
Cited by
-
Components of the Gs signaling cascade exhibit distinct changes in mobility and membrane domain localization upon β2 -adrenergic receptor activation.Traffic. 2020 Apr;21(4):324-332. doi: 10.1111/tra.12724. Traffic. 2020. PMID: 32096320 Free PMC article.
-
Membrane phosphoinositides regulate GPCR-β-arrestin complex assembly and dynamics.Cell. 2022 Nov 23;185(24):4560-4573.e19. doi: 10.1016/j.cell.2022.10.018. Epub 2022 Nov 10. Cell. 2022. PMID: 36368322 Free PMC article.
-
MagIC-Cryo-EM: Structural determination on magnetic beads for scarce macromolecules in heterogeneous samples.bioRxiv [Preprint]. 2025 Jan 7:2024.01.21.576499. doi: 10.1101/2024.01.21.576499. bioRxiv. 2025. PMID: 38328033 Free PMC article. Preprint.
-
miR-7 Regulates GLP-1-Mediated Insulin Release by Targeting β-Arrestin 1.Cells. 2020 Jul 6;9(7):1621. doi: 10.3390/cells9071621. Cells. 2020. PMID: 32640511 Free PMC article.
-
Accelerating GPCR Drug Discovery With Conformation-Stabilizing VHHs.Front Mol Biosci. 2022 May 23;9:863099. doi: 10.3389/fmolb.2022.863099. eCollection 2022. Front Mol Biosci. 2022. PMID: 35677880 Free PMC article. Review.
References
-
- Lefkowitz RJ, Stadel JM & Caron MG Adenylate cyclase-coupled beta-adrenergic receptors: structure and mechanisms of activation and desensitization. Annu Rev Biochem 52, 159–86 (1983). - PubMed
-
- Oakley RH, Laporte SA, Holt JA, Barak LS & Caron MG Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. Journal of Biological Chemistry 274, 32248–32257 (1999). - PubMed
-
- Oakley RH, Laporte SA, Holt JA, Caron MG & Barak LS Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem 275, 17201–10 (2000). - PubMed
Methods-only References
-
- Mastronarde DN Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol 152, 36–51 (2005). - PubMed
-
- Suloway C et al. Automated molecular microscopy: the new Leginon system. J Struct Biol 151, 41–60 (2005). - PubMed
-
- Tegunov D & Cramer P Real-time cryo-EM data pre-processing with Warp. BioRxiv (2018).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

