Serrated Colorectal Cancer: The Road Less Travelled?
- PMID: 31735291
- PMCID: PMC6894428
- DOI: 10.1016/j.trecan.2019.09.004
Serrated Colorectal Cancer: The Road Less Travelled?
Abstract
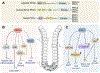
Studies of colorectal cancer (CRC) originating through the conventional adenoma-carcinoma sequence have provided insight into the molecular mechanisms controlling its initiation and progression. Less is known about the alternative 'serrated' pathway, which has been associated with BRAF mutation and microsatellite instability. Recent transcriptomics approaches to classify human CRC revealed that mesenchymal and/or desmoplastic features combined with an immunosuppressive microenvironment are key determinants of CRC with the poorest prognosis. Importantly, these aggressive CRCs harbor the characteristics of serrated tumors, suggesting that initiation through this alternative pathway determines how aggressive the CRC becomes. Here, we review recent evidence on how serrated carcinogenesis contributes to the subtype of CRC with the poorest prognosis.
Keywords: atypical PKC; colorectal cancer; immune checkpoint therapy; mesenchymal; microenvironment; serrated.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Distinct features between MLH1-methylated and unmethylated colorectal carcinomas with the CpG island methylator phenotype: implications in the serrated neoplasia pathway.Oncotarget. 2016 Mar 22;7(12):14095-111. doi: 10.18632/oncotarget.7374. Oncotarget. 2016. PMID: 26883113 Free PMC article.
-
TGFβ-Responsive Stromal Activation Occurs Early in Serrated Colorectal Carcinogenesis.Int J Mol Sci. 2024 Apr 24;25(9):4626. doi: 10.3390/ijms25094626. Int J Mol Sci. 2024. PMID: 38731846 Free PMC article.
-
Genetic characteristics of mitochondrial DNA was associated with colorectal carcinogenesis and its prognosis.PLoS One. 2015 Mar 3;10(3):e0118612. doi: 10.1371/journal.pone.0118612. eCollection 2015. PLoS One. 2015. PMID: 25734426 Free PMC article.
-
CpG Island Methylator Phenotype-High Colorectal Cancers and Their Prognostic Implications and Relationships with the Serrated Neoplasia Pathway.Gut Liver. 2017 Jan 15;11(1):38-46. doi: 10.5009/gnl15535. Gut Liver. 2017. PMID: 27885175 Free PMC article. Review.
-
[Colorectal serrated lesions: current insight on their role in colorectal carcinogenesis].Duodecim. 2010;126(17):2002-11. Duodecim. 2010. PMID: 21053517 Review. Finnish.
Cited by
-
From Crypts to Cancer: A Holistic Perspective on Colorectal Carcinogenesis and Therapeutic Strategies.Int J Mol Sci. 2024 Aug 30;25(17):9463. doi: 10.3390/ijms25179463. Int J Mol Sci. 2024. PMID: 39273409 Free PMC article. Review.
-
Protein kinase Cλ/ι in cancer: a contextual balance of time and signals.Trends Cell Biol. 2022 Dec;32(12):1023-1034. doi: 10.1016/j.tcb.2022.04.002. Epub 2022 Apr 29. Trends Cell Biol. 2022. PMID: 35501226 Free PMC article. Review.
-
Microbiome distinctions between the CRC carcinogenic pathways.Gut Microbes. 2021 Jan-Dec;13(1):1854641. doi: 10.1080/19490976.2020.1854641. Epub 2021 Jan 15. Gut Microbes. 2021. PMID: 33446008 Free PMC article. Review.
-
Biology and Therapeutic Targets of Colorectal Serrated Adenocarcinoma; Clues for a Histologically Based Treatment against an Aggressive Tumor.Int J Mol Sci. 2020 Mar 14;21(6):1991. doi: 10.3390/ijms21061991. Int J Mol Sci. 2020. PMID: 32183342 Free PMC article. Review.
-
Quadruplicate Synchronous Adenocarcinoma of the Colon with Distant Metastases-Long-Term Molecular Follow-Up by KRAS and TP53 Mutational Profiling.Diagnostics (Basel). 2020 Jun 16;10(6):407. doi: 10.3390/diagnostics10060407. Diagnostics (Basel). 2020. PMID: 32560038 Free PMC article.
References
-
- Siegel RL et al. (2019) Cancer statistics, 2019. CA Cancer J Clin 69 (1), 7–34. - PubMed
-
- Fearon ER and Vogelstein B (1990) A genetic model for colorectal tumorigenesis. Cell 61 (5), 759–67. - PubMed
-
- Jass JR (2001) Serrated route to colorectal cancer: back street or super highway? J Pathol 193 (3), 283–5. - PubMed
-
- Vogelstein B et al. (1988) Genetic alterations during colorectal-tumor development. N Engl J Med 319 (9), 525–32. - PubMed
-
- Jass JR and Smith M (1992) Sialic acid and epithelial differentiation in colorectal polyps and cancer--a morphological, mucin and lectin histochemical study. Pathology 24 (4), 233–42. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

