Cytomegalovirus Humoral Response Against Epithelial Cell Entry-Mediated Infection in the Primary Infection Setting After Hematopoietic Cell Transplantation
- PMID: 31734696
- PMCID: PMC7137890
- DOI: 10.1093/infdis/jiz596
Cytomegalovirus Humoral Response Against Epithelial Cell Entry-Mediated Infection in the Primary Infection Setting After Hematopoietic Cell Transplantation
Abstract
Background: The influence of humoral immunity on the prevention of primary cytomegalovirus (CMV) infection after hematopoietic cell transplantation (HCT) is poorly understood.
Methods: To determine whether neutralizing antibodies (nAbs) against CMV pentameric complex (PC)-mediated epithelial cell entry decrease CMV infection after HCT, samples were analyzed from a randomized controlled trial of CMV intravenous immunoglobulin (IVIG) prophylaxis. Weekly serum from 61 CMV donor-positive/recipient-negative (D+/R-) HCT patients (33 control, 28 CMV IVIG) was tested using a PC-entry nAb assay and quantitative CMV polymerase chain reaction (PCR).
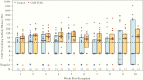
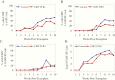
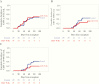
Results: There was a trend toward higher weekly PC-entry nAb titers (P = .07) and decreased CMV infection by PCR at viral load cutoffs of ≥1000 and ≥10 000 IU/mL in the CMV IVIG arm. High nAb titers were not significantly protective against CMV infection later after HCT in both study arms. Among CMV-infected patients, each log2 increase in nAb titer was associated with an average 0.2 log10 decrease in concurrent CMV viral load after infection (P = .001; adjusted for study arm).
Conclusions: This study provides initial support that CMV IVIG prophylaxis moderately enhances PC-entry nAB activity in D+/R- HCT recipients.
Keywords: CMV; cytomegalovirus; hematopoietic cell transplantation; neutralizing antibodies; pentameric complex.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
Cytomegalovirus pre-emptive therapy after hematopoietic stem cell transplantation in the era of real-time quantitative PCR: comparison with recipients of solid organ transplants.Transpl Infect Dis. 2016 Jun;18(3):405-14. doi: 10.1111/tid.12542. Epub 2016 Jun 9. Transpl Infect Dis. 2016. PMID: 27061703
-
Randomized, placebo-controlled, double-blind study of a cytomegalovirus-specific monoclonal antibody (MSL-109) for prevention of cytomegalovirus infection after allogeneic hematopoietic stem cell transplantation.Biol Blood Marrow Transplant. 2001;7(6):343-51. doi: 10.1016/s1083-8791(01)80005-7. Biol Blood Marrow Transplant. 2001. PMID: 11464977 Clinical Trial.
-
Letermovir for primary and secondary cytomegalovirus prevention in allogeneic hematopoietic cell transplant recipients: Real-world experience.Transpl Infect Dis. 2019 Dec;21(6):e13187. doi: 10.1111/tid.13187. Epub 2019 Oct 21. Transpl Infect Dis. 2019. PMID: 31585500 Free PMC article.
-
Cytomegalovirus Infection in Solid Organ and Hematopoietic Cell Transplantation: State of the Evidence.J Infect Dis. 2020 Mar 5;221(Suppl 1):S23-S31. doi: 10.1093/infdis/jiz454. J Infect Dis. 2020. PMID: 32134486 Free PMC article. Review.
-
The Immunology of Posttransplant CMV Infection: Potential Effect of CMV Immunoglobulins on Distinct Components of the Immune Response to CMV.Transplantation. 2016 Mar;100 Suppl 3(Suppl 3):S11-8. doi: 10.1097/TP.0000000000001095. Transplantation. 2016. PMID: 26900990 Free PMC article. Review.
Cited by
-
Cytomegalovirus immunity in high-risk liver transplant recipients following preemptive antiviral therapy versus prophylaxis.JCI Insight. 2024 Sep 24;9(18):e180115. doi: 10.1172/jci.insight.180115. JCI Insight. 2024. PMID: 39099206 Free PMC article. Clinical Trial.
-
Progress and Challenges in the Prevention, Diagnosis, and Management of Cytomegalovirus Infection in Transplantation.Clin Microbiol Rev. 2020 Oct 28;34(1):e00043-19. doi: 10.1128/CMR.00043-19. Print 2020 Dec 16. Clin Microbiol Rev. 2020. PMID: 33115722 Free PMC article. Review.
-
Cytomegalovirus-specific neutralizing antibodies effectively prevent uncontrolled infection after allogeneic hematopoietic stem cell transplantation.iScience. 2022 Sep 5;25(10):105065. doi: 10.1016/j.isci.2022.105065. eCollection 2022 Oct 21. iScience. 2022. PMID: 36147955 Free PMC article.
-
Diagnosis and treatment for the early stage of cytomegalovirus infection during hematopoietic stem cell transplantation.Front Immunol. 2022 Sep 23;13:971156. doi: 10.3389/fimmu.2022.971156. eCollection 2022. Front Immunol. 2022. PMID: 36211358 Free PMC article. Review.

