Inhibitory Effects of Osthole on Human Breast Cancer Cell Progression via Induction of Cell Cycle Arrest, Mitochondrial Dysfunction, and ER Stress
- PMID: 31731635
- PMCID: PMC6893636
- DOI: 10.3390/nu11112777
Inhibitory Effects of Osthole on Human Breast Cancer Cell Progression via Induction of Cell Cycle Arrest, Mitochondrial Dysfunction, and ER Stress
Abstract
Background: Breast cancer is the most commonly diagnosed cancer and the second leading cause of cancer death in women. Although, recently, the number of pathological studies of breast cancer have increased, it is necessary to identify a novel compound that targets multiple signaling pathways involved in breast cancer.
Methods: The effects of osthole on cell viability, apoptosis, mitochondria-mediated apoptosis, production of reactive oxygen species (ROS), and endoplasmic reticulum (ER) stress proteins of BT-474 and MCF-7 breast cancer cell lines were investigated. Signal transduction pathways in both cells in response to osthole were determined by western blot analyses.
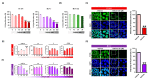
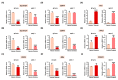
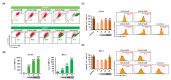
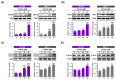
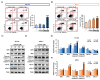
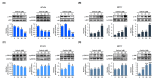
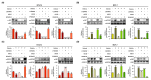
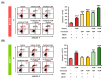
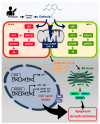
Results: Here, we demonstrated that osthole inhibited cellular proliferation and induced cell cycle arrest through modulation of cell cycle regulatory genes in BT-474 and MCF-7 cells. Additionally, osthole induced loss of mitochondrial membrane potential (MMP), intracellular calcium imbalance, and ER stress. Moreover, osthole induced apoptosis by activating the pro-apoptotic protein, Bax, in both cell lines. Osthole regulated phosphorylation of signaling proteins such as Akt and ERK1/2 in human breast cancer cells. Furthermore, osthole-induced activation of JNK protein-mediated apoptosis in both cell lines.
Conclusions: Collectively, the results of the present study indicated that osthole may ameliorate breast cancer and can be a promising therapeutic agent for treatment of breast cancer.
Keywords: ER stress; MMP depolarization; apoptosis; breast cancer; calcium imbalance; osthole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Chrysophanol selectively represses breast cancer cell growth by inducing reactive oxygen species production and endoplasmic reticulum stress via AKT and mitogen-activated protein kinase signal pathways.Toxicol Appl Pharmacol. 2018 Dec 1;360:201-211. doi: 10.1016/j.taap.2018.10.010. Epub 2018 Oct 6. Toxicol Appl Pharmacol. 2018. PMID: 30300626
-
Bufalin Induces Apoptosis of Human Osteosarcoma U-2 OS Cells through Endoplasmic Reticulum Stress, Caspase- and Mitochondria-Dependent Signaling Pathways.Molecules. 2017 Mar 10;22(3):437. doi: 10.3390/molecules22030437. Molecules. 2017. PMID: 28287444 Free PMC article.
-
Coumestrol induces mitochondrial dysfunction by stimulating ROS production and calcium ion influx into mitochondria in human placental choriocarcinoma cells.Mol Hum Reprod. 2017 Nov 1;23(11):786-802. doi: 10.1093/molehr/gax052. Mol Hum Reprod. 2017. PMID: 29040664
-
Mitochondrial Stress Response and Cancer.Trends Cancer. 2020 Aug;6(8):688-701. doi: 10.1016/j.trecan.2020.04.009. Epub 2020 May 22. Trends Cancer. 2020. PMID: 32451306 Free PMC article. Review.
-
Molecular mechanisms of hepatic apoptosis.Cell Death Dis. 2014 Jan 16;5(1):e996. doi: 10.1038/cddis.2013.499. Cell Death Dis. 2014. PMID: 24434519 Free PMC article. Review.
Cited by
-
KIFC3 Promotes Proliferation, Migration, and Invasion in Colorectal Cancer via PI3K/AKT/mTOR Signaling Pathway.Front Genet. 2022 Jun 22;13:848926. doi: 10.3389/fgene.2022.848926. eCollection 2022. Front Genet. 2022. PMID: 35812733 Free PMC article.
-
Osthole induces necroptosis via ROS overproduction in glioma cells.FEBS Open Bio. 2021 Feb;11(2):456-467. doi: 10.1002/2211-5463.13069. Epub 2021 Jan 19. FEBS Open Bio. 2021. PMID: 33350608 Free PMC article.
-
Osthole induces apoptosis of the HT-29 cells via endoplasmic reticulum stress and autophagy.Oncol Lett. 2021 Oct;22(4):726. doi: 10.3892/ol.2021.12987. Epub 2021 Aug 10. Oncol Lett. 2021. PMID: 34429766 Free PMC article.
-
4-Hydroxyderricin Promotes Apoptosis and Cell Cycle Arrest through Regulating PI3K/AKT/mTOR Pathway in Hepatocellular Cells.Foods. 2021 Aug 29;10(9):2036. doi: 10.3390/foods10092036. Foods. 2021. PMID: 34574146 Free PMC article.
-
Osthole exhibits an antitumor effect in retinoblastoma through inhibiting the PI3K/AKT/mTOR pathway via regulating the hsa_circ_0007534/miR-214-3p axis.Pharm Biol. 2022 Dec;60(1):417-426. doi: 10.1080/13880209.2022.2032206. Pharm Biol. 2022. PMID: 35175172 Free PMC article.
References
-
- Winters S., Martin C., Murphy D., Shokar N.K. Chapter One—Breast Cancer Epidemiology, Prevention, and Screening. In: Lakshmanaswamy R., editor. Progress in Molecular Biology and Translational Science. Volume 151. Academic Press; Cambridge, MA, USA: 2017. pp. 1–32. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

