Biomechanical assessment of remote and postinfarction scar remodeling following myocardial infarction
- PMID: 31727993
- PMCID: PMC6856121
- DOI: 10.1038/s41598-019-53351-7
Biomechanical assessment of remote and postinfarction scar remodeling following myocardial infarction
Abstract
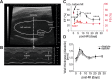
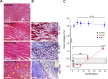
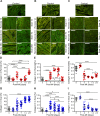
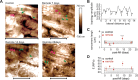
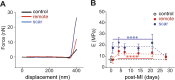
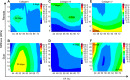
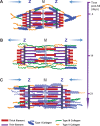
The importance of collagen remodeling following myocardial infarction (MI) is extensively investigated, but little is known on the biomechanical impact of fibrillar collagen on left ventricle post-MI. We aim to identify the significant effects of the biomechanics of types I, III, and V collagen on physio-pathological changes of murine hearts leading to heart failure. Immediately post-MI, heart reduces its function (EF = 40.94 ± 2.12%) while sarcomeres' dimensions are unchanged. Strikingly, as determined by immunohistochemistry staining, type V collagen fraction significantly grows in remote and scar for sustaining de novo-types I and III collagen fibers' assembly while hindering their enzymatic degradation. Thereafter, the compensatory heart function (EF = 63.04 ± 3.16%) associates with steady development of types I and III collagen in a stiff remote (12.79 ± 1.09 MPa) and scar (22.40 ± 1.08 MPa). In remote, the soft de novo-type III collagen uncoils preventing further expansion of elongated sarcomeres (2.7 ± 0.3 mm). Once the compensatory mechanisms are surpassed, the increased turnover of stiff type I collagen (>50%) lead to a pseudo-stable biomechanical regime of the heart (≅9 MPa) with reduced EF (50.55 ± 3.25%). These end-characteristics represent the common scenario evidenced in patients suffering from heart failure after MI. Our pre-clinical data advances the understanding of the cause of heart failure induced in patients with extended MI.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Type V Collagen in Scar Tissue Regulates the Size of Scar after Heart Injury.Cell. 2020 Aug 6;182(3):545-562.e23. doi: 10.1016/j.cell.2020.06.030. Epub 2020 Jul 3. Cell. 2020. PMID: 32621799 Free PMC article.
-
Expression of Gq alpha and PLC-beta in scar and border tissue in heart failure due to myocardial infarction.Circulation. 1998 Mar 10;97(9):892-9. doi: 10.1161/01.cir.97.9.892. Circulation. 1998. PMID: 9521338
-
Role for MicroRNA-21 in atrial profibrillatory fibrotic remodeling associated with experimental postinfarction heart failure.Circ Arrhythm Electrophysiol. 2012 Oct;5(5):1027-35. doi: 10.1161/CIRCEP.112.973214. Epub 2012 Aug 26. Circ Arrhythm Electrophysiol. 2012. PMID: 22923342
-
Clinical aspects of left ventricular diastolic function assessed by Doppler echocardiography following acute myocardial infarction.Dan Med Bull. 2001 Nov;48(4):199-210. Dan Med Bull. 2001. PMID: 11767125 Review.
-
Physiological Implications of Myocardial Scar Structure.Compr Physiol. 2015 Sep 20;5(4):1877-909. doi: 10.1002/cphy.c140067. Compr Physiol. 2015. PMID: 26426470 Free PMC article. Review.
Cited by
-
Aminoacylase-1 plays a key role in myocardial fibrosis and the therapeutic effects of 20(S)-ginsenoside Rg3 in mouse heart failure.Acta Pharmacol Sin. 2022 Aug;43(8):2003-2015. doi: 10.1038/s41401-021-00830-1. Epub 2021 Dec 16. Acta Pharmacol Sin. 2022. PMID: 34916608 Free PMC article.
-
Fourier analysis of collagen bundle orientation in myocardial infarction scars.Histochem Cell Biol. 2022 Nov;158(5):471-483. doi: 10.1007/s00418-022-02132-x. Epub 2022 Aug 10. Histochem Cell Biol. 2022. PMID: 35948735 Free PMC article.
-
Inflammatory Cytokines Alter Mesenchymal Stem Cell Mechanosensing and Adhesion on Stiffened Infarct Heart Tissue After Myocardial Infarction.Front Cell Dev Biol. 2020 Oct 23;8:583700. doi: 10.3389/fcell.2020.583700. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33195229 Free PMC article.
-
Tropoelastin Improves Post-Infarct Cardiac Function.Circ Res. 2023 Jan 6;132(1):72-86. doi: 10.1161/CIRCRESAHA.122.321123. Epub 2022 Dec 1. Circ Res. 2023. PMID: 36453283 Free PMC article.
-
Lack of expression of miR-29a/b1 impairs bladder function in male mice.Dis Model Mech. 2023 Jun 1;16(6):dmm050054. doi: 10.1242/dmm.050054. Epub 2023 Jun 7. Dis Model Mech. 2023. PMID: 37283037 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

