TTC5 mediates autoregulation of tubulin via mRNA degradation
- PMID: 31727855
- PMCID: PMC6942541
- DOI: 10.1126/science.aaz4352
TTC5 mediates autoregulation of tubulin via mRNA degradation
Abstract
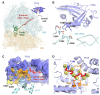
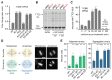
Tubulins play crucial roles in cell division, intracellular traffic, and cell shape. Tubulin concentration is autoregulated by feedback control of messenger RNA (mRNA) degradation via an unknown mechanism. We identified tetratricopeptide protein 5 (TTC5) as a tubulin-specific ribosome-associating factor that triggers cotranslational degradation of tubulin mRNAs in response to excess soluble tubulin. Structural analysis revealed that TTC5 binds near the ribosome exit tunnel and engages the amino terminus of nascent tubulins. TTC5 mutants incapable of ribosome or nascent tubulin interaction abolished tubulin autoregulation and showed chromosome segregation defects during mitosis. Our findings show how a subset of mRNAs can be targeted for coordinated degradation by a specificity factor that recognizes the nascent polypeptides they encode.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures


Comment in
-
Gene expression regulated by RNA stability.Science. 2020 Jan 3;367(6473):29. doi: 10.1126/science.aba0713. Science. 2020. PMID: 31896707 No abstract available.
-
Make or break: the ribosome as a regulator of mRNA decay.Cell Res. 2020 Mar;30(3):195-196. doi: 10.1038/s41422-019-0271-3. Cell Res. 2020. PMID: 31913358 Free PMC article. No abstract available.
Similar articles
-
Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
"It Is a Big Spider Web of Things": Sensory Experiences of Autistic Adults in Public Spaces.Autism Adulthood. 2023 Dec 1;5(4):411-422. doi: 10.1089/aut.2022.0024. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116051 Free PMC article.
-
Structural basis of mRNA decay by the human exosome-ribosome supercomplex.Nature. 2024 Nov;635(8037):237-242. doi: 10.1038/s41586-024-08015-6. Epub 2024 Oct 9. Nature. 2024. PMID: 39385025 Free PMC article.
-
Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
Cited by
-
Monitoring of XRN4 Targets Reveals the Importance of Cotranslational Decay during Arabidopsis Development.Plant Physiol. 2020 Nov;184(3):1251-1262. doi: 10.1104/pp.20.00942. Epub 2020 Sep 10. Plant Physiol. 2020. PMID: 32913043 Free PMC article.
-
miR-31-mediated local translation at the mitotic spindle is important for early development.Development. 2024 Sep 1;151(17):dev202619. doi: 10.1242/dev.202619. Epub 2024 Sep 5. Development. 2024. PMID: 39250531
-
TBA-7 is not a microtubule-destabilizing tubulin.Mol Biol Cell. 2021 Jun 1;32(12):1145-1146. doi: 10.1091/mbc.E21-04-0157. Mol Biol Cell. 2021. PMID: 34043426 Free PMC article. No abstract available.
-
The Expression and Function of Tubulin Isotypes in Caenorhabditis elegans.Front Cell Dev Biol. 2022 Mar 24;10:860065. doi: 10.3389/fcell.2022.860065. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35399537 Free PMC article. Review.
-
Low Intensity Ultrasound as an Antidote to Taxane/Paclitaxel-induced Cytotoxicity.J Cancer. 2022 Apr 18;13(7):2362-2373. doi: 10.7150/jca.71263. eCollection 2022. J Cancer. 2022. PMID: 35517405 Free PMC article. Review.
References
-
- Desai A, Mitchison TJ. Microtubule Polymerization Dynamics. Annu Rev Cell Dev Biol. 1997;13:83–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

