Inhibition of phosphatidylinositol 3-kinase by PX-866 suppresses temozolomide-induced autophagy and promotes apoptosis in glioblastoma cells
- PMID: 31726966
- PMCID: PMC6854621
- DOI: 10.1186/s10020-019-0116-z
Inhibition of phosphatidylinositol 3-kinase by PX-866 suppresses temozolomide-induced autophagy and promotes apoptosis in glioblastoma cells
Abstract
Background: Temozolomide (TMZ) is the most commonly used chemotherapeutic agent used to treat glioblastoma (GBM), which causes significant DNA damage to highly proliferative cells. Our observations have added to accumulating evidence that TMZ induces stress-responsive cellular programs known to promote cell survival, including autophagy. As such, targeting these survival pathways may represent new vulnerabilities of GBM after treatment with TMZ.
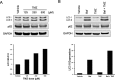
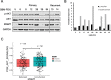
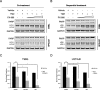
Methods: Using the T98G human glioma cell line, we assessed the molecular signaling associated with TMZ treatment, the cellular consequences of using the pan-PI3K inhibitor PX-866, and performed clonogenic assays to determine the effect sequential treatment of TMZ and PX-866 had on colony formation. Additionally, we also use subcutaneous GBM patient derived xenograft (PDX) tumors to show relative LC3 protein expression and correlations between survival pathways and molecular markers which dictate clinical responsiveness to TMZ.
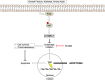
Results: Here, we report that TMZ can induce autophagic flux in T98G glioma cells. GBM patient-derived xenograft (PDX) tumors treated with TMZ also display an increase in the autophagosome marker LC3 II. Additionally, O6-methylguanine-DNA-methyltransferase (MGMT) expression correlates with PI3K/AKT activity, suggesting that patients with inherent resistance to TMZ (MGMT-high) would benefit from PI3K/AKT inhibitors in addition to TMZ. Accordingly, we have identified that the blood-brain barrier (BBB) penetrant pan-PI3K inhibitor, PX-866, is an early-stage inhibitor of autophagic flux, while maintaining its ability to inhibit PI3K/AKT signaling in glioma cells. Lastly, due to the induction of autophagic flux by TMZ, we provide evidence for sequential treatment of TMZ followed by PX-866, rather than combined co-treatment, as a means to shut down autophagy-induced survival in GBM cells and to enhance apoptosis.
Conclusions: The understanding of how TMZ induces survival pathways, such as autophagy, may offer new therapeutic vulnerabilities and opportunities to use sequential inhibition of alternate pro-survival pathways that regulate autophagy. As such, identification of additional ways to inhibit TMZ-induced autophagy could enhance the efficacy of TMZ.
Keywords: Autophagy; Bafilomycin; Glioblastoma (GBM); PI3K; PX-866; Temozolomide (TMZ).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Micro-RNA29b enhances the sensitivity of glioblastoma multiforme cells to temozolomide by promoting autophagy.Anat Rec (Hoboken). 2021 Feb;304(2):342-352. doi: 10.1002/ar.24400. Epub 2020 Apr 23. Anat Rec (Hoboken). 2021. PMID: 32275350
-
Enhanced cytotoxic effect of radiation and temozolomide in malignant glioma cells: targeting PI3K-AKT-mTOR signaling, HSP90 and histone deacetylases.BMC Cancer. 2014 Jan 13;14:17. doi: 10.1186/1471-2407-14-17. BMC Cancer. 2014. PMID: 24418474 Free PMC article.
-
Simvastatin Induces Unfolded Protein Response and Enhances Temozolomide-Induced Cell Death in Glioblastoma Cells.Cells. 2020 Oct 22;9(11):2339. doi: 10.3390/cells9112339. Cells. 2020. PMID: 33105603 Free PMC article.
-
Importance of Autophagy Regulation in Glioblastoma with Temozolomide Resistance.Cells. 2024 Aug 11;13(16):1332. doi: 10.3390/cells13161332. Cells. 2024. PMID: 39195222 Free PMC article. Review.
-
An Interplay between Senescence, Apoptosis and Autophagy in Glioblastoma Multiforme-Role in Pathogenesis and Therapeutic Perspective.Int J Mol Sci. 2018 Mar 17;19(3):889. doi: 10.3390/ijms19030889. Int J Mol Sci. 2018. PMID: 29562589 Free PMC article. Review.
Cited by
-
Quiescin Sulfhydryl Oxidase 1 Regulates the Proliferation, Migration and Invasion of Human Glioblastoma Cells via PI3K/Akt Pathway.Onco Targets Ther. 2020 Jun 17;13:5721-5729. doi: 10.2147/OTT.S255941. eCollection 2020. Onco Targets Ther. 2020. PMID: 32606784 Free PMC article.
-
Mechanisms of temozolomide resistance in glioblastoma - a comprehensive review.Cancer Drug Resist. 2021;4(1):17-43. doi: 10.20517/cdr.2020.79. Epub 2021 Mar 19. Cancer Drug Resist. 2021. PMID: 34337348 Free PMC article.
-
The PYK2 inhibitor PF-562271 enhances the effect of temozolomide on tumor growth in a C57Bl/6-Gl261 mouse glioma model.J Neurooncol. 2023 Feb;161(3):593-604. doi: 10.1007/s11060-023-04260-3. Epub 2023 Feb 15. J Neurooncol. 2023. PMID: 36790653 Free PMC article.
-
PD-L1 Inhibitor Regulates the miR-33a-5p/PTEN Signaling Pathway and Can Be Targeted to Sensitize Glioblastomas to Radiation.Front Oncol. 2020 May 27;10:821. doi: 10.3389/fonc.2020.00821. eCollection 2020. Front Oncol. 2020. PMID: 32537433 Free PMC article.
-
Crosstalk between metabolism and cell death in tumorigenesis.Mol Cancer. 2024 Apr 4;23(1):71. doi: 10.1186/s12943-024-01977-1. Mol Cancer. 2024. PMID: 38575922 Free PMC article. Review.
References
-
- Buccarelli M, Marconi M, Pacioni S, De Pascalis I, D'Alessandris QG, Martini M, Ascione B, Malorni W, Larocca LM, Pallini R, et al. Inhibition of autophagy increases susceptibility of glioblastoma stem cells to temozolomide by igniting ferroptosis. Cell Death Dis. 2018;9(8):841. doi: 10.1038/s41419-018-0864-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

