Structural biology of thermoTRPV channels
- PMID: 31726322
- PMCID: PMC6893863
- DOI: 10.1016/j.ceca.2019.102106
Structural biology of thermoTRPV channels
Abstract
Essential for physiology, transient receptor potential (TRP) channels constitute a large and diverse family of cation channels functioning as cellular sensors responding to a vast array of physical and chemical stimuli. Detailed understanding of the inner workings of TRP channels has been hampered by a lack of atomic structures, though structural biology of TRP channels has been an enthusiastic endeavor since their molecular identification two decades ago. These multi-domain integral membrane proteins, exhibiting complex polymodal gating behavior, have been a challenge for traditional X-ray crystallography, which requires formation of well-ordered protein crystals. X-ray structures remain limited to a few TRP channel proteins to date. Fortunately, recent breakthroughs in single-particle cryo-electron microscopy (cryo-EM) have enabled rapid growth of the number of TRP channel structures, providing tremendous insights into channel gating and regulation mechanisms and serving as foundations for further mechanistic investigations. This brief review focuses on recent exciting developments in structural biology of a subset of TRP channels, the calcium-permeable, non-selective and thermosensitive vanilloid subfamily of TRP channels (TRPV1-4), and the permeation and gating mechanisms revealed by structures.
Keywords: Cryo-EM; Crystallography; Ion channel; TRP channel; TRPV; ThermoTRPV.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures

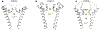

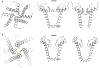
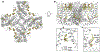
Similar articles
-
Determining the Crystal Structure of TRPV6.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 14. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 14. PMID: 30299652 Free Books & Documents. Review.
-
Structural insights into the gating mechanisms of TRPV channels.Cell Calcium. 2020 May;87:102168. doi: 10.1016/j.ceca.2020.102168. Epub 2020 Jan 24. Cell Calcium. 2020. PMID: 32004816 Free PMC article. Review.
-
Dawning of a new era in TRP channel structural biology by cryo-electron microscopy.Pflugers Arch. 2018 Feb;470(2):213-225. doi: 10.1007/s00424-018-2107-2. Epub 2018 Jan 17. Pflugers Arch. 2018. PMID: 29344776 Review.
-
X-ray crystallography of TRP channels.Channels (Austin). 2018 Jan 1;12(1):137-152. doi: 10.1080/19336950.2018.1457898. Channels (Austin). 2018. PMID: 29589513 Free PMC article. Review.
-
Structural and Evolutionary Insights Point to Allosteric Regulation of TRP Ion Channels.Acc Chem Res. 2019 Jun 18;52(6):1643-1652. doi: 10.1021/acs.accounts.9b00075. Epub 2019 May 31. Acc Chem Res. 2019. PMID: 31149807 Free PMC article.
Cited by
-
TRPV1: Structure, Endogenous Agonists, and Mechanisms.Int J Mol Sci. 2020 May 12;21(10):3421. doi: 10.3390/ijms21103421. Int J Mol Sci. 2020. PMID: 32408609 Free PMC article. Review.
-
Ligand-Binding Sites in Vanilloid-Subtype TRP Channels.Front Pharmacol. 2022 May 16;13:900623. doi: 10.3389/fphar.2022.900623. eCollection 2022. Front Pharmacol. 2022. PMID: 35652046 Free PMC article. Review.
-
Structural Pharmacology of TRPV4 Antagonists.Adv Sci (Weinh). 2024 Jul;11(25):e2401583. doi: 10.1002/advs.202401583. Epub 2024 Apr 24. Adv Sci (Weinh). 2024. PMID: 38659239 Free PMC article.
-
Sample preparation of the human TRPA1 ion channel for cryo-EM studies.Methods Enzymol. 2021;653:75-87. doi: 10.1016/bs.mie.2020.12.018. Epub 2021 Jan 27. Methods Enzymol. 2021. PMID: 34099182 Free PMC article.
-
Sequence and structural conservation reveal fingerprint residues in TRP channels.Elife. 2022 Jun 10;11:e73645. doi: 10.7554/eLife.73645. Elife. 2022. PMID: 35686986 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

