Mechanisms of GABAB receptor enhancement of extrasynaptic GABAA receptor currents in cerebellar granule cells
- PMID: 31723152
- PMCID: PMC6853962
- DOI: 10.1038/s41598-019-53087-4
Mechanisms of GABAB receptor enhancement of extrasynaptic GABAA receptor currents in cerebellar granule cells
Abstract
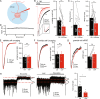
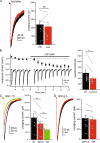
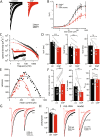
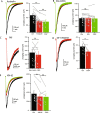
Many neurons, including cerebellar granule cells, exhibit a tonic GABA current mediated by extrasynaptic GABAA receptors. This current is a critical regulator of firing and the target of many clinically relevant compounds. Using a combination of patch clamp electrophysiology and photolytic uncaging of RuBi-GABA we show that GABAB receptors are tonically active and enhance extrasynaptic GABAA receptor currents in cerebellar granule cells. This enhancement is not associated with meaningful changes in GABAA receptor potency, mean channel open-time, open probability, or single-channel current. However, there was a significant (~40%) decrease in the number of channels participating in the GABA uncaging current and an increase in receptor desensitization. Furthermore, we find that adenylate cyclase, PKA, CaMKII, and release of Ca2+ from intracellular stores are necessary for modulation of GABAA receptors. Overall, this work reveals crosstalk between postsynaptic GABAA and GABAB receptors and identifies the signaling pathways and mechanisms involved.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Biphasic modulation of parallel fibre synaptic transmission by co-activation of presynaptic GABAA and GABAB receptors in mice.J Physiol. 2016 Jul 1;594(13):3651-66. doi: 10.1113/JP272124. Epub 2016 May 29. J Physiol. 2016. PMID: 27061582 Free PMC article.
-
Balancing tonic and phasic inhibition in hypothalamic corticotropin-releasing hormone neurons.J Physiol. 2018 May 15;596(10):1919-1929. doi: 10.1113/JP275588. Epub 2018 Mar 14. J Physiol. 2018. PMID: 29419884 Free PMC article.
-
GABA-B receptors enhance GABA-A receptor currents by modulation of membrane trafficking in dentate gyrus granule cells.Neurosci Lett. 2022 Mar 16;773:136481. doi: 10.1016/j.neulet.2022.136481. Epub 2022 Jan 29. Neurosci Lett. 2022. PMID: 35104617
-
Bridging the cleft at GABA synapses in the brain.Trends Neurosci. 1994 Dec;17(12):517-25. doi: 10.1016/0166-2236(94)90155-4. Trends Neurosci. 1994. PMID: 7532336 Review.
-
α6-Containing GABAA Receptors: Functional Roles and Therapeutic Potentials.Pharmacol Rev. 2022 Jan;74(1):238-270. doi: 10.1124/pharmrev.121.000293. Pharmacol Rev. 2022. PMID: 35017178 Review.
Cited by
-
Disinhibition Is an Essential Network Motif Coordinated by GABA Levels and GABA B Receptors.Int J Mol Sci. 2024 Jan 22;25(2):1340. doi: 10.3390/ijms25021340. Int J Mol Sci. 2024. PMID: 38279339 Free PMC article. Review.
-
Reduced Activation of the Synaptic-Type GABAA Receptor Following Prolonged Exposure to Low Concentrations of Agonists: Relationship between Tonic Activity and Desensitization.Mol Pharmacol. 2020 Dec;98(6):762-769. doi: 10.1124/molpharm.120.000088. Epub 2020 Sep 25. Mol Pharmacol. 2020. PMID: 32978327 Free PMC article.
-
Increased Voluntary Alcohol Consumption in Mice Lacking GABAB(1) Is Associated With Functional Changes in Hippocampal GABAA Receptors.Front Behav Neurosci. 2022 Jun 9;16:893835. doi: 10.3389/fnbeh.2022.893835. eCollection 2022. Front Behav Neurosci. 2022. PMID: 35755407 Free PMC article.
-
Tonic GABAergic inhibition, via GABAA receptors containing αβƐ subunits, regulates excitability of ventral tegmental area dopamine neurons.Eur J Neurosci. 2021 Mar;53(6):1722-1737. doi: 10.1111/ejn.15133. Epub 2021 Feb 14. Eur J Neurosci. 2021. PMID: 33522050 Free PMC article.
-
Impact of aging on the GABAB receptor-mediated connectome.bioRxiv [Preprint]. 2024 Aug 2:2024.07.31.606013. doi: 10.1101/2024.07.31.606013. bioRxiv. 2024. PMID: 39131332 Free PMC article. Preprint.
References
-
- Brickley SG, Cull-Candy SG, Farrant M. Single-channel properties of synaptic and extrasynaptic GABAA receptors suggest differential targeting of receptor subtypes. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1999;19:2960–2973. doi: 10.1523/JNEUROSCI.19-08-02960.1999. - DOI - PMC - PubMed
-
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

